Chemistry 1011
Chemistry 1011
Chemistry 1011
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
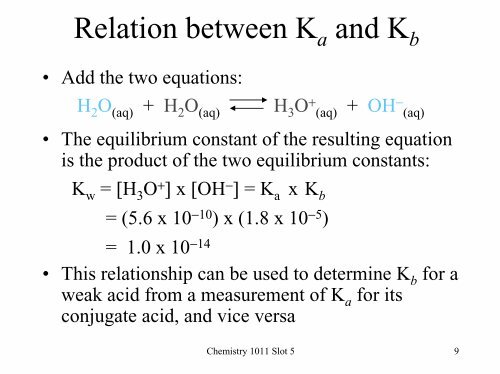
Relation between K a and K b• Add the two equations:H 2 O (aq) + H 2 O (aq) H 3 O + (aq) + OH − (aq)• The equilibrium constant of the resulting equationis the product of the two equilibrium constants:K w = [H 3 O + ] x [OH − ] = K a x K b= (5.6 x 10 −10 ) x (1.8 x 10 −5 )= 1.0 x 10 −14• This relationship can be used to determine K b for aweak acid from a measurement of K a for itsconjugate acid, and vice versa<strong>Chemistry</strong> <strong>1011</strong> Slot 5 9