Chemistry 1011
Chemistry 1011
Chemistry 1011
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
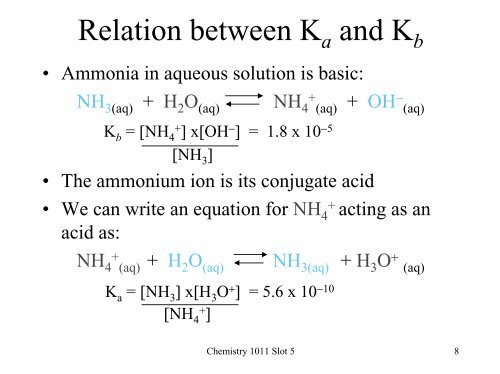
Relation between K a and K b• Ammonia in aqueous solution is basic:NH 3(aq) + H 2 O (aq) NH+ 4 (aq) + OH − (aq)K b = [NH 4+ ] x[OH − ] = 1.8 x 10 −5[NH 3 ]• The ammonium ion is its conjugate acid• We can write an equation for NH 4+ acting as anacid as:NH+ + 4 (aq) H 2 O (aq) NH 3(aq) + H 3 O + (aq)K a = [NH 3 ] x[H 3 O + ] = 5.6 x 10 −10[NH 4+ ]<strong>Chemistry</strong> <strong>1011</strong> Slot 5 8