impurities in new drug products
impurities in new drug products
impurities in new drug products
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
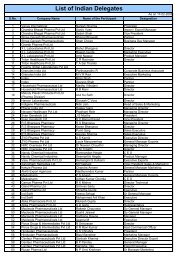
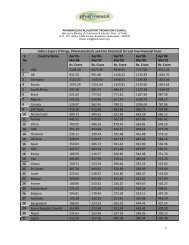
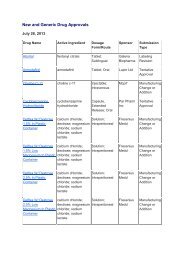
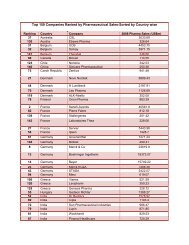
Impurities <strong>in</strong> New Drug ProductsIllustration of Thresholds for Report<strong>in</strong>g, Identification, and Qualification ofDegradation Products <strong>in</strong> New Drug Products as a Function ofMaximum Daily Dose 1Percentage of Drug Substance1.000.800.600.400.20Report<strong>in</strong>gIdentificationQualification0.000 500 1000 1500 2000 2500Maximum Daily Dose Expressed <strong>in</strong> mg of Drug SubstanceExpanded Scale:1.00Percentage of Drug Substance0.800.600.400.20Report<strong>in</strong>gIdentificationQualification0.000 5 10 15 20Maximum Daily Dose Expressed <strong>in</strong> mg of Drug Substance1 Note : Actual threshold values should be taken from the preced<strong>in</strong>g table <strong>in</strong> this attachment.8