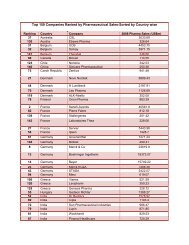
impurities in new drug products
impurities in new drug products
impurities in new drug products
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
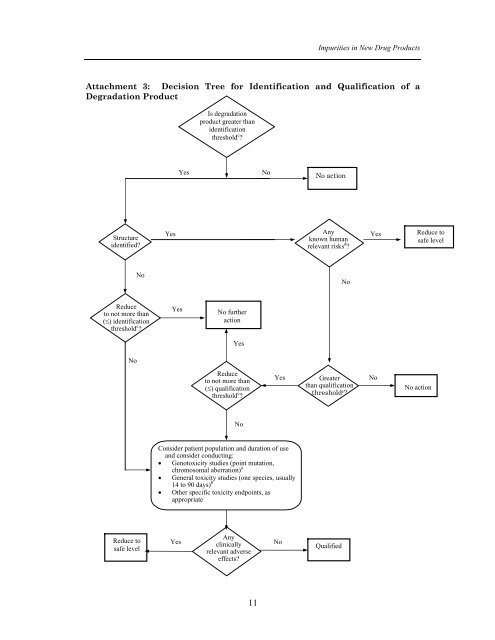
Impurities <strong>in</strong> New Drug ProductsAttachment 3: Decision Tree for Identification and Qualification of aDegradation ProductIs degradationproduct greater thanidentificationthreshold c ?YesNoNo actionStructureidentified?YesAnyknown humanrelevant risks d ?YesReduce tosafe levelNoNoReduceto not more than(≤) identificationthreshold c ?NoYesNo furtheractionYesReduceto not more than(≤) qualificationthreshold c ?YesGreaterthan qualificationthreshold c ?NoNo actionNoConsider patient population and duration of useand consider conduct<strong>in</strong>g:• Genotoxicity studies (po<strong>in</strong>t mutation,chromosomal aberration) a• General toxicity studies (one species, usually14 to 90 days) b• Other specific toxicity endpo<strong>in</strong>ts, asappropriateReduce tosafe levelYesAnycl<strong>in</strong>icallyrelevant adverseeffects?NoQualified11