What is the theoretical background of conductometry?
What is the theoretical background of conductometry?
What is the theoretical background of conductometry?
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
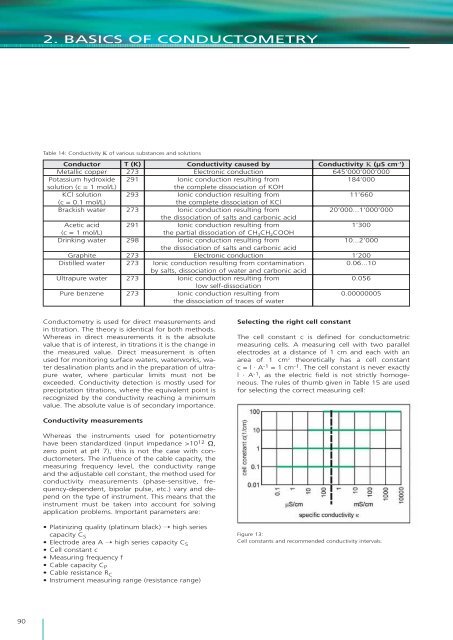
2. BASICS OF CONDUCTOMETRYTable 14: Conductivity Κ <strong>of</strong> various substances and solutionsConductorMetallic copperPotassium hydroxidesolution (c = 1 mol/L)KCl solution(c = 0.1 mol/L)Brack<strong>is</strong>h waterAcetic acid(c = 1 mol/L)Drinking waterGraphiteD<strong>is</strong>tilled waterUltrapure waterPure benzeneT (K)273291293273291298273273273273Conductivity caused byElectronic conductionIonic conduction resulting from<strong>the</strong> complete d<strong>is</strong>sociation <strong>of</strong> KOHIonic conduction resulting from<strong>the</strong> complete d<strong>is</strong>sociation <strong>of</strong> KClIonic conduction resulting from<strong>the</strong> d<strong>is</strong>sociation <strong>of</strong> salts and carbonic acidIonic conduction resulting from<strong>the</strong> partial d<strong>is</strong>sociation <strong>of</strong> CH 3 CH 2 COOHIonic conduction resulting from<strong>the</strong> d<strong>is</strong>sociation <strong>of</strong> salts and carbonic acidElectronic conductionIonic conduction resulting from contaminationby salts, d<strong>is</strong>sociation <strong>of</strong> water and carbonic acidIonic conduction resulting fromlow self-d<strong>is</strong>sociationIonic conduction resulting from<strong>the</strong> d<strong>is</strong>sociation <strong>of</strong> traces <strong>of</strong> waterConductivity Κ (μS cm –1 )645’000’000’000184’00011’66020’000...1’000’0001’30010...2’0001’2000.06...100.0560.00000005Conductometry <strong>is</strong> used for direct measurements andin titration. The <strong>the</strong>ory <strong>is</strong> identical for both methods.Whereas in direct measurements it <strong>is</strong> <strong>the</strong> absolutevalue that <strong>is</strong> <strong>of</strong> interest, in titrations it <strong>is</strong> <strong>the</strong> change in<strong>the</strong> measured value. Direct measurement <strong>is</strong> <strong>of</strong>tenused for monitoring surface waters, waterworks, waterdesalination plants and in <strong>the</strong> preparation <strong>of</strong> ultrapurewater, where particular limits must not beexceeded. Conductivity detection <strong>is</strong> mostly used forprecipitation titrations, where <strong>the</strong> equivalent point <strong>is</strong>recognized by <strong>the</strong> conductivity reaching a minimumvalue. The absolute value <strong>is</strong> <strong>of</strong> secondary importance.Selecting <strong>the</strong> right cell constantThe cell constant c <strong>is</strong> defined for conductometricmeasuring cells. A measuring cell with two parallelelectrodes at a d<strong>is</strong>tance <strong>of</strong> 1 cm and each with anarea <strong>of</strong> 1 cm 2 <strong>the</strong>oretically has a cell constantc = l · A -1 = 1 cm -1 . The cell constant <strong>is</strong> never exactlyl · A -1 , as <strong>the</strong> electric field <strong>is</strong> not strictly homogeneous.The rules <strong>of</strong> thumb given in Table 15 are usedfor selecting <strong>the</strong> correct measuring cell:Conductivity measurementsWhereas <strong>the</strong> instruments used for potentiometryhave been standardized (input impedance >10 12 Ω,zero point at pH 7), th<strong>is</strong> <strong>is</strong> not <strong>the</strong> case with conductometers.The influence <strong>of</strong> <strong>the</strong> cable capacity, <strong>the</strong>measuring frequency level, <strong>the</strong> conductivity rangeand <strong>the</strong> adjustable cell constant, <strong>the</strong> method used forconductivity measurements (phase-sensitive, frequency-dependent,bipolar pulse, etc.) vary and dependon <strong>the</strong> type <strong>of</strong> instrument. Th<strong>is</strong> means that <strong>the</strong>instrument must be taken into account for solvingapplication problems. Important parameters are:• Platinizing quality (platinum black) ➝ high seriescapacity C S• Electrode area A ➝ high series capacity C S• Cell constant c• Measuring frequency f• Cable capacity C P• Cable res<strong>is</strong>tance R C• Instrument measuring range (res<strong>is</strong>tance range)Figure 13:Cell constants and recommended conductivity intervals.90