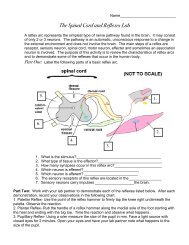
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
<strong>The</strong> <strong>Oxidizing</strong> <strong>Power</strong> <strong>of</strong> <strong>Bleach</strong>Purpose:To determine the weight percent <strong>of</strong> NaClO in commercial bleach, through anoxidation-reduction titration (iodometric analysis).NOTE – notice that this is not the same purpose that was provided in the lab.Procedure:1. Rinse the buret with distilled water2. Rinse the buret with two portions <strong>of</strong> sodium thiosulfate3. Fill the buret with 0.1 M sodium thiosulfate4. Obtain 25 mL <strong>of</strong> dilute bleach solution in a 125 mL Erlenmeyer flask5. Add approximately 1 g <strong>of</strong> KI to the dilute bleach solution6. Add 5 mL <strong>of</strong> glacial acetic acid to the flask (this is done in the hood)7. Titrate the solution until it turns clear yellow8. Add starch solution drop-wise to the flask until the solution is deep blue9. Continue titrating until the solution turns clear and colorless10. Read sodium thiosulfate volume in buret11. Pour waste in fume hood sink12. Repeat titration two more timesData: See attachedCalculations: See attachedSources <strong>of</strong> Error:One source <strong>of</strong> error in this experiment is that the original bleach solution was old,therefore some <strong>of</strong> the NaClO in the bleach had decomposed over time and the weightpercent will be less than the 6% listed on the commercial bleach container. Anothersource <strong>of</strong> error is the use <strong>of</strong> a graduated cylinder to measure the volume <strong>of</strong> bleachsolution used. It was difficult to determine exactly 25 mL <strong>of</strong> bleach solution. <strong>The</strong>calculations in step five would be affected if either more or less solution was actuallydelivered. For example, if more than 25 mL’s <strong>of</strong> bleach solution was used during thetitration; the calculated weight percent <strong>of</strong> NaClO would have been larger than the actualvalue.Conclusions:Iodometric analysis was performed in order to determine the weight percent <strong>of</strong>NaClO in commercial bleach. Potassium iodide was added to a dilute solution <strong>of</strong>bleach and glacial acetic acid. A redox reaction occurred between the hypochlorite ionand the iodide ion to produce iodine. <strong>The</strong> iodine was then titrated against sodiumthiosulfate. Calculations involving the volume <strong>of</strong> 0.100 M sodium thiosulfate allowedfor the determination <strong>of</strong> the weight percent <strong>of</strong> NaClO in commercial bleach. <strong>The</strong>experimental value was calculated to be 2.98%, which is a 50.3% difference from the
published value. Two sources <strong>of</strong> error that account for this large difference are listedabove. Another error in the lab was the execution <strong>of</strong> the titration. During the firsttitration our group overshot the end point, which eventually affected the value <strong>of</strong> theweight percent <strong>of</strong> NaClO in commercial bleach. Overall, I learned that titrations arenot just for acid-base reactions, and that it can be used in context <strong>of</strong> redox reactions.Also, the actual chemical reaction that occurred during the titration did not involve theion for which we wanted to obtain information. We were able to determine the value <strong>of</strong>sodium hypochlorite in commercial bleach using two balanced equations andstoichiometric calculations.