Advances in the stereoselective synthesis of antifungal agents and ...
Advances in the stereoselective synthesis of antifungal agents and ...
Advances in the stereoselective synthesis of antifungal agents and ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
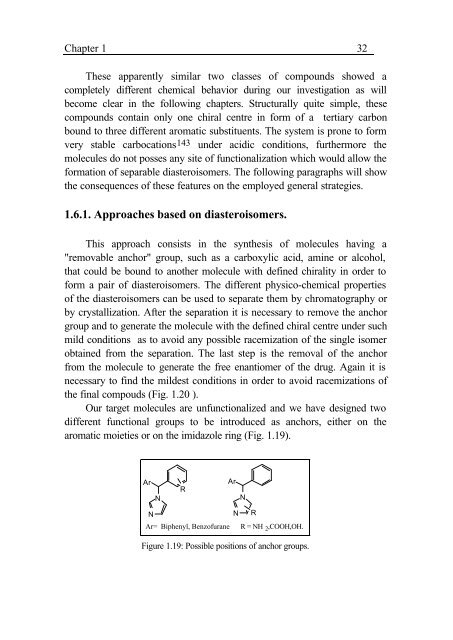
Chapter 1 32These apparently similar two classes <strong>of</strong> compounds showed acompletely different chemical behavior dur<strong>in</strong>g our <strong>in</strong>vestigation as willbecome clear <strong>in</strong> <strong>the</strong> follow<strong>in</strong>g chapters. Structurally quite simple, <strong>the</strong>secompounds conta<strong>in</strong> only one chiral centre <strong>in</strong> form <strong>of</strong> a tertiary carbonbound to three different aromatic substituents. The system is prone to formvery stable carbocations 143 under acidic conditions, fur<strong>the</strong>rmore <strong>the</strong>molecules do not posses any site <strong>of</strong> functionalization which would allow <strong>the</strong>formation <strong>of</strong> separable diasteroisomers. The follow<strong>in</strong>g paragraphs will show<strong>the</strong> consequences <strong>of</strong> <strong>the</strong>se features on <strong>the</strong> employed general strategies.1.6.1. Approaches based on diasteroisomers.This approach consists <strong>in</strong> <strong>the</strong> syn<strong>the</strong>sis <strong>of</strong> molecules hav<strong>in</strong>g a"removable anchor" group, such as a carboxylic acid, am<strong>in</strong>e or alcohol,that could be bound to ano<strong>the</strong>r molecule with def<strong>in</strong>ed chirality <strong>in</strong> order t<strong>of</strong>orm a pair <strong>of</strong> diasteroisomers. The different physico-chemical properties<strong>of</strong> <strong>the</strong> diasteroisomers can be used to separate <strong>the</strong>m by chromatography orby crystallization. After <strong>the</strong> separation it is necessary to remove <strong>the</strong> anchorgroup <strong>and</strong> to generate <strong>the</strong> molecule with <strong>the</strong> def<strong>in</strong>ed chiral centre under suchmild conditions as to avoid any possible racemization <strong>of</strong> <strong>the</strong> s<strong>in</strong>gle isomerobta<strong>in</strong>ed from <strong>the</strong> separation. The last step is <strong>the</strong> removal <strong>of</strong> <strong>the</strong> anchorfrom <strong>the</strong> molecule to generate <strong>the</strong> free enantiomer <strong>of</strong> <strong>the</strong> drug. Aga<strong>in</strong> it isnecessary to f<strong>in</strong>d <strong>the</strong> mildest conditions <strong>in</strong> order to avoid racemizations <strong>of</strong><strong>the</strong> f<strong>in</strong>al compouds (Fig. 1.20 ).Our target molecules are unfunctionalized <strong>and</strong> we have designed twodifferent functional groups to be <strong>in</strong>troduced as anchors, ei<strong>the</strong>r on <strong>the</strong>aromatic moieties or on <strong>the</strong> imidazole r<strong>in</strong>g (Fig. 1.19).ArArRNNNN RAr= Biphenyl, Benz<strong>of</strong>urane R = NH 2 ,COOH,OH.Figure 1.19: Possible positions <strong>of</strong> anchor groups.