Advances in the stereoselective synthesis of antifungal agents and ...
Advances in the stereoselective synthesis of antifungal agents and ...
Advances in the stereoselective synthesis of antifungal agents and ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
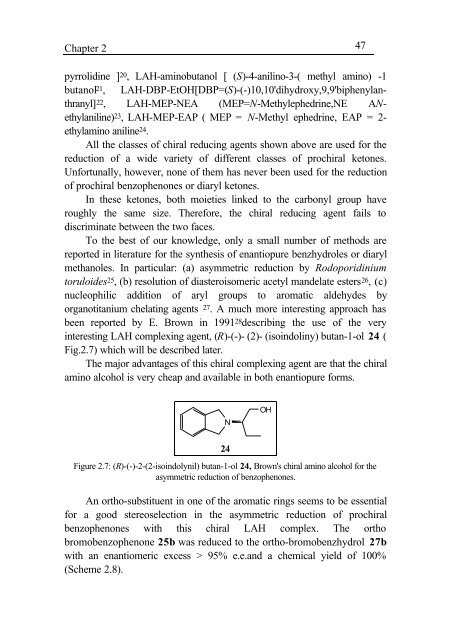
Chapter 2 47pyrrolid<strong>in</strong>e ] 20 , LAH-am<strong>in</strong>obutanol [ (S)-4-anil<strong>in</strong>o-3-( methyl am<strong>in</strong>o) -1butanol 21 , LAH-DBP-EtOH[DBP=(S)-(-)10,10'dihydroxy,9,9'biphenylanthranyl]22 , LAH-MEP-NEA (MEP=N-Methylephedr<strong>in</strong>e,NE ANethylanil<strong>in</strong>e)23 , LAH-MEP-EAP ( MEP = N-Methyl ephedr<strong>in</strong>e, EAP = 2-ethylam<strong>in</strong>o anil<strong>in</strong>e 24 .All <strong>the</strong> classes <strong>of</strong> chiral reduc<strong>in</strong>g <strong>agents</strong> shown above are used for <strong>the</strong>reduction <strong>of</strong> a wide variety <strong>of</strong> different classes <strong>of</strong> prochiral ketones.Unfortunally, however, none <strong>of</strong> <strong>the</strong>m has never been used for <strong>the</strong> reduction<strong>of</strong> prochiral benzophenones or diaryl ketones.In <strong>the</strong>se ketones, both moieties l<strong>in</strong>ked to <strong>the</strong> carbonyl group haveroughly <strong>the</strong> same size. Therefore, <strong>the</strong> chiral reduc<strong>in</strong>g agent fails todiscrim<strong>in</strong>ate between <strong>the</strong> two faces.To <strong>the</strong> best <strong>of</strong> our knowledge, only a small number <strong>of</strong> methods arereported <strong>in</strong> literature for <strong>the</strong> syn<strong>the</strong>sis <strong>of</strong> enantiopure benzhydroles or diarylmethanoles. In particular: (a) asymmetric reduction by Rodoporid<strong>in</strong>iumtoruloides 25 , (b) resolution <strong>of</strong> diasteroisomeric acetyl m<strong>and</strong>elate esters 26 , (c)nucleophilic addition <strong>of</strong> aryl groups to aromatic aldehydes byorganotitanium chelat<strong>in</strong>g <strong>agents</strong> 27 . A much more <strong>in</strong>terest<strong>in</strong>g approach hasbeen reported by E. Brown <strong>in</strong> 1991 28 describ<strong>in</strong>g <strong>the</strong> use <strong>of</strong> <strong>the</strong> very<strong>in</strong>terest<strong>in</strong>g LAH complex<strong>in</strong>g agent, (R)-(-)- (2)- (iso<strong>in</strong>dol<strong>in</strong>y) butan-1-ol 24 (Fig.2.7) which will be described later.The major advantages <strong>of</strong> this chiral complex<strong>in</strong>g agent are that <strong>the</strong> chiralam<strong>in</strong>o alcohol is very cheap <strong>and</strong> available <strong>in</strong> both enantiopure forms.NOH24Figure 2.7: (R)-(-)-2-(2-iso<strong>in</strong>dolynil) butan-1-ol 24, Brown's chiral am<strong>in</strong>o alcohol for <strong>the</strong>asymmetric reduction <strong>of</strong> benzophenones.An ortho-substituent <strong>in</strong> one <strong>of</strong> <strong>the</strong> aromatic r<strong>in</strong>gs seems to be essentialfor a good stereoselection <strong>in</strong> <strong>the</strong> asymmetric reduction <strong>of</strong> prochiralbenzophenones with this chiral LAH complex. The orthobromobenzophenone 25b was reduced to <strong>the</strong> ortho-bromobenzhydrol 27bwith an enantiomeric excess > 95% e.e.<strong>and</strong> a chemical yield <strong>of</strong> 100%(Scheme 2.8).