Investigational
novartisnovartisnovartis
novartisnovartisnovartis
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
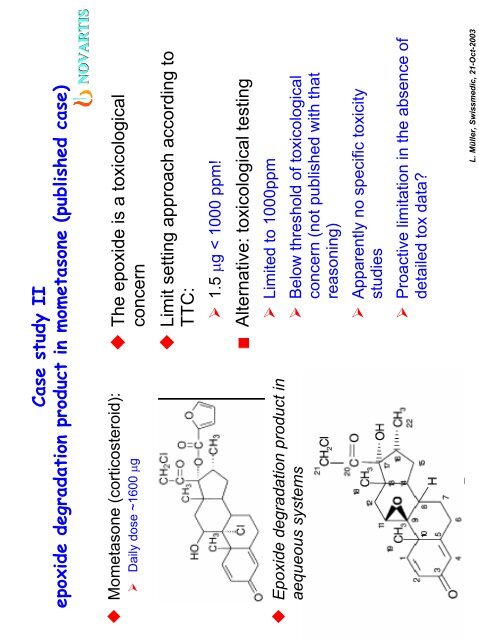
Case study II<br />
epoxide degradation product in mometasone (published case)<br />
NOVARTIS<br />
Mometasone (corticosteroid):<br />
‣ Daily dose ~1600 µg<br />
Epoxide degradation product in<br />
aequeous systems<br />
The epoxide is a toxicological<br />
concern<br />
Limit setting approach according to<br />
TTC:<br />
‣ 1.5 µg < 1000 ppm!<br />
Alternative: toxicological testing<br />
‣ Limited to 1000ppm<br />
‣ Below threshold of toxicological<br />
concern (not published with that<br />
reasoning)<br />
‣ Apparently no specific toxicity<br />
studies<br />
‣ Proactive limitation in the absence of<br />
detailed tox data?<br />
L. Müller, Swissmedic, 21-Oct-2003