DRUGS REPORTS
oFF-Patents - iagim
oFF-Patents - iagim
- No tags were found...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
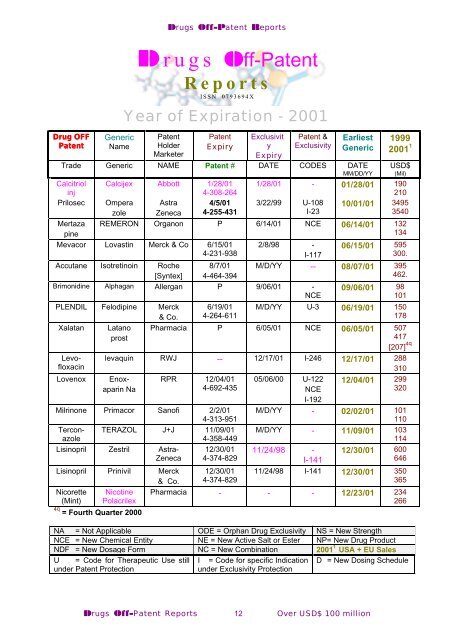
Drug OFF<br />
Generic Patent<br />
Patent<br />
Name Holder<br />
Marketer<br />
Drugs Off-Patent Reports<br />
Drugs Off-Patent<br />
Reports<br />
ISSN 0793694X<br />
Year of Expiration - 2001<br />
Patent<br />
Expiry<br />
Exclusivit<br />
y<br />
Expiry<br />
Patent &<br />
Exclusivity<br />
Earliest<br />
Generic<br />
Trade Generic NAME Patent # DATE CODES DATE<br />
MM/DD/YY<br />
Calcitriol Calcijex Abbott 1/28/01<br />
inj<br />
4-308-264<br />
Prilosec<br />
Mertaza<br />
pine<br />
Ompera<br />
zole<br />
Astra<br />
Zeneca<br />
4/5/01<br />
4-255-431<br />
1999<br />
2001 1<br />
USD$<br />
(Mil)<br />
1/28/01 - 01/28/01 190<br />
210<br />
3/22/99 U-108 10/01/01 3495<br />
I-23<br />
3540<br />
REMERON Organon P 6/14/01 NCE 06/14/01 132<br />
134<br />
Mevacor Lovastin Merck & Co 6/15/01<br />
4-231-938<br />
Accutane Isotretinoin Roche 8/7/01<br />
[Syntex] 4-464-394<br />
2/8/98 -<br />
I-117<br />
Brimonidine Alphagan Allergan P 9/06/01 -<br />
NCE<br />
PLENDIL Felodipine Merck<br />
& Co.<br />
Xalatan<br />
6/19/01<br />
4-264-611<br />
06/15/01 595<br />
300.<br />
M/D/YY -- 08/07/01 395<br />
462.<br />
09/06/01 98<br />
101<br />
M/D/YY U-3 06/19/01 150<br />
178<br />
Latano<br />
prost<br />
Pharmacia P 6/05/01 NCE 06/05/01 507<br />
417<br />
[207] 4q<br />
levaquin RWJ -- 12/17/01 I-246 12/17/01 288<br />
310<br />
Levofloxacin<br />
Lovenox<br />
Enoxaparin<br />
Na<br />
RPR 12/04/01<br />
4-692-435<br />
Milrinone Primacor Sanofi 2/2/01<br />
4-313-951<br />
Terconazole<br />
TERAZOL J+J 11/09/01<br />
4-358-449<br />
Lisinopril Zestril Astra-<br />
Zeneca<br />
Lisinopril Prinivil Merck<br />
& Co.<br />
Nicorette Nicotine<br />
(Mint) Polacrilex<br />
4q<br />
= Fourth Quarter 2000<br />
12/30/01<br />
4-374-829<br />
12/30/01<br />
4-374-829<br />
05/06/00 U-122<br />
NCE<br />
I-192<br />
12/04/01 299<br />
320<br />
M/D/YY - 02/02/01 101<br />
110<br />
M/D/YY - 11/09/01 103<br />
114<br />
11/24/98 - 12/30/01 600<br />
I-141<br />
646<br />
11/24/98 I-141 12/30/01 350<br />
365<br />
Pharmacia - - - 12/23/01 234<br />
266<br />
NA = Not Applicable ODE = Orphan Drug Exclusivity NS = New Strength<br />
NCE = New Chemical Entity NE = New Active Salt or Ester NP= New Drug Product<br />
NDF = New Dosage Form NC = New Combination 2001 1 USA + EU Sales<br />
U = Code for Therapeutic Use still I = Code for specific Indication D = New Dosing Schedule<br />
under Patent Protection<br />
under Exclusivity Protection<br />
Drugs Off-Patent Reports 12 Over USD$ 100 million