DRUGS REPORTS
oFF-Patents - iagim
oFF-Patents - iagim
- No tags were found...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
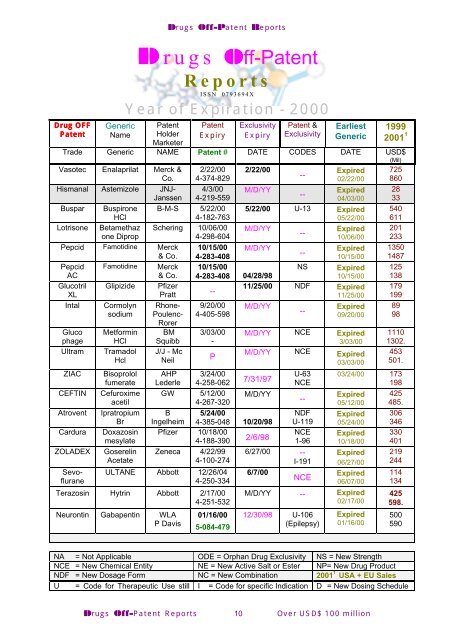
Drugs Off-Patent Reports<br />
Drugs Off-Patent<br />
Reports<br />
ISSN 0793694X<br />
Year of Expiration - 2000<br />
Drug OFF<br />
Generic Patent Patent Exclusivity Patent & Earliest 1999<br />
Patent<br />
Name Holder Expiry Expiry Exclusivity Generic 2001 1<br />
Marketer<br />
Trade Generic NAME Patent # DATE CODES DATE USD$<br />
(Mil)<br />
2/22/00<br />
Vasotec Enalaprilat Merck &<br />
Co.<br />
Hismanal Astemizole JNJ-<br />
Janssen<br />
Buspar Buspirone<br />
HCl<br />
Lotrisone<br />
Betamethaz<br />
one Diprop<br />
2/22/00<br />
4-374-829<br />
4/3/00<br />
4-219-559<br />
B-M-S 5/22/00<br />
4-182-763<br />
Schering 10/06/00<br />
4-298-604<br />
Gluco<br />
phage<br />
Ultram<br />
ZIAC<br />
CEFTIN<br />
Atrovent<br />
Cardura<br />
ZOLADEX<br />
Pepcid Famotidine Merck<br />
& Co.<br />
10/15/00<br />
4-283-408<br />
Pepcid Famotidine Merck 10/15/00<br />
AC<br />
& Co. 4-283-408 04/28/98<br />
Glucotril Glipizide Pfizer<br />
XL<br />
Pratt --<br />
Intal Cormolyn Rhone- 9/20/00<br />
sodium Poulenc- 4-405-598<br />
Sevoflurane<br />
Metformin<br />
HCl<br />
Tramadol<br />
Hcl<br />
Bisoprolol<br />
fumerate<br />
Cefuroxime<br />
acetil<br />
Ipratropium<br />
Br<br />
Doxazosin<br />
mesylate<br />
Goserelin<br />
Acetate<br />
Rorer<br />
BM<br />
Squibb<br />
J/J - Mc<br />
Neil<br />
3/03/00<br />
-<br />
P<br />
AHP 3/24/00<br />
Lederle 4-258-062 7/31/97<br />
GW 5/12/00 M/D/YY<br />
4-267-320<br />
B 5/24/00<br />
Ingelheim 4-385-048 10/20/98<br />
Pfizer 10/18/00<br />
4-188-390 2/6/98<br />
Zeneca 4/22/99<br />
4-100-274<br />
ULTANE Abbott 12/26/04<br />
4-250-334<br />
Terazosin Hytrin Abbott 2/17/00<br />
4-251-532<br />
Neurontin Gabapentin WLA<br />
P Davis<br />
01/16/00<br />
5-084-479<br />
--<br />
Expired<br />
02/22/00<br />
M/D/YY<br />
Expired<br />
--<br />
04/03/00<br />
5/22/00 U-13 Expired<br />
05/22/00<br />
M/D/YY<br />
Expired<br />
--<br />
10/06/00<br />
M/D/YY<br />
Expired<br />
--<br />
10/15/00<br />
NS Expired<br />
10/15/00<br />
11/25/00 NDF Expired<br />
11/25/00<br />
M/D/YY<br />
Expired<br />
--<br />
09/20/00<br />
M/D/YY NCE Expired<br />
3/03/00<br />
M/D/YY NCE Expired<br />
03/03/00<br />
U-63<br />
NCE<br />
--<br />
NDF<br />
U-119<br />
NCE<br />
1-96<br />
6/27/00 --<br />
I-191<br />
6/7/00<br />
NCE<br />
725<br />
860<br />
28<br />
33<br />
540<br />
611<br />
201<br />
233<br />
1350<br />
1487<br />
125<br />
138<br />
179<br />
199<br />
89<br />
98<br />
1110<br />
1302.<br />
453<br />
501.<br />
03/24/00 173<br />
198<br />
Expired<br />
05/12/00<br />
Expired<br />
05/24/00<br />
Expired<br />
10/18/00<br />
Expired<br />
06/27/00<br />
Expired<br />
06/07/00<br />
M/D/YY -- Expired<br />
02/17/00<br />
12/30/98 U-106<br />
(Epilepsy)<br />
Expired<br />
01/16/00<br />
425<br />
485.<br />
306<br />
346<br />
330<br />
401<br />
219<br />
244<br />
114<br />
134<br />
425<br />
598.<br />
500<br />
590<br />
NA = Not Applicable ODE = Orphan Drug Exclusivity NS = New Strength<br />
NCE = New Chemical Entity NE = New Active Salt or Ester NP= New Drug Product<br />
NDF = New Dosage Form NC = New Combination 2001 1 USA + EU Sales<br />
U = Code for Therapeutic Use still I = Code for specific Indication D = New Dosing Schedule<br />
Drugs Off-Patent Reports 10 Over USD$ 100 million