DRUGS REPORTS
oFF-Patents - iagim
oFF-Patents - iagim
- No tags were found...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
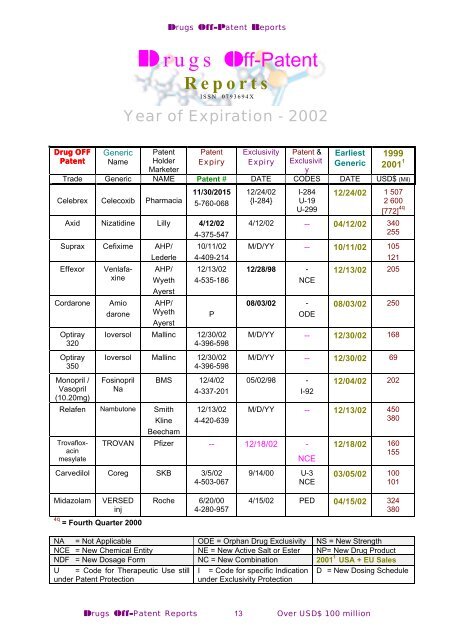
Drugs Off-Patent Reports<br />
Drugs Off-Patent<br />
Reports<br />
ISSN 0793694X<br />
Year of Expiration - 2002<br />
Drug OFF<br />
Generic Patent Patent Exclusivity Patent & Earliest 1999<br />
Patent<br />
Name Holder Expiry Expiry Exclusivit Generic 2001 1<br />
Marketer<br />
y<br />
Trade Generic NAME Patent # DATE CODES DATE USD$ (Mil)<br />
Celebrex Celecoxib Pharmacia<br />
11/30/2015<br />
5-760-068<br />
Optiray<br />
320<br />
Optiray<br />
350<br />
Monopril /<br />
Vasopril<br />
(10.20mg)<br />
Ioversol Mallinc 12/30/02<br />
4-396-598<br />
Ioversol Mallinc 12/30/02<br />
4-396-598<br />
Fosinopril<br />
Na<br />
BMS 12/4/02<br />
4-337-201<br />
12/24/02<br />
{I-284}<br />
I-284<br />
U-19<br />
U-299<br />
12/24/02 1 507<br />
2 600<br />
[772] 4q<br />
4/12/02 -- 04/12/02 340<br />
255<br />
M/D/YY -- 10/11/02 105<br />
121<br />
12/28/98 - 12/13/02 205<br />
NCE<br />
08/03/02 -<br />
ODE<br />
08/03/02 250<br />
M/D/YY -- 12/30/02 168<br />
M/D/YY -- 12/30/02 69<br />
05/02/98 -<br />
I-92<br />
Axid Nizatidine Lilly 4/12/02<br />
4-375-547<br />
Suprax Cefixime AHP/ 10/11/02<br />
Lederle 4-409-214<br />
Effexor Venlafaxine<br />
AHP/ 12/13/02<br />
Wyeth 4-535-186<br />
Ayerst<br />
Cordarone Amio AHP/<br />
darone Wyeth P<br />
Ayerst<br />
Relafen Nambutone Smith<br />
Kline<br />
Beecham<br />
12/13/02<br />
4-420-639<br />
Trovafloxacin<br />
TROVAN Pfizer -- 12/18/02 -<br />
mesylate<br />
NCE<br />
Carvedilol Coreg SKB 3/5/02<br />
4-503-067<br />
12/04/02 202<br />
M/D/YY -- 12/13/02 450<br />
380<br />
9/14/00 U-3<br />
NCE<br />
12/18/02 160<br />
155<br />
03/05/02 100<br />
101<br />
Midazolam VERSED<br />
inj<br />
4q<br />
= Fourth Quarter 2000<br />
Roche 6/20/00<br />
4-280-957<br />
4/15/02 PED 04/15/02 324<br />
380<br />
NA = Not Applicable ODE = Orphan Drug Exclusivity NS = New Strength<br />
NCE = New Chemical Entity NE = New Active Salt or Ester NP= New Drug Product<br />
NDF = New Dosage Form NC = New Combination 2001 1 USA + EU Sales<br />
U = Code for Therapeutic Use still I = Code for specific Indication D = New Dosing Schedule<br />
under Patent Protection<br />
under Exclusivity Protection<br />
Drugs Off-Patent Reports 13 Over USD$ 100 million