DRUGS REPORTS
oFF-Patents - iagim
oFF-Patents - iagim
- No tags were found...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
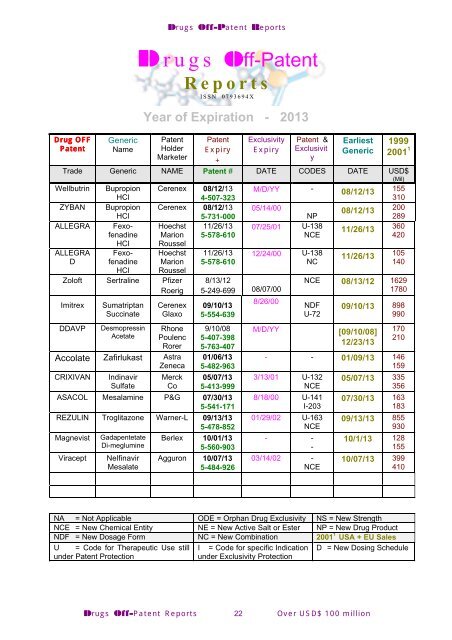
Drug OFF<br />
Generic Patent<br />
Patent<br />
Name Holder<br />
Marketer<br />
Drugs Off-Patent Reports<br />
Drugs Off-Patent<br />
Reports<br />
ISSN 0793694X<br />
Year of Expiration - 2013<br />
Patent<br />
Expiry<br />
+<br />
Exclusivity<br />
Expiry<br />
Patent &<br />
Exclusivit<br />
y<br />
Earliest<br />
Generic<br />
1999<br />
2001 1<br />
Trade Generic NAME Patent # DATE CODES DATE USD$<br />
(Mil)<br />
Wellbutrin Bupropion Cerenex 08/12/13 M/D/YY -<br />
08/12/13<br />
155<br />
HCl<br />
4-507-323<br />
310<br />
ZYBAN Bupropion Cerenex 08/12/13 05/14/00<br />
08/12/13<br />
200<br />
HCl<br />
5-731-000<br />
NP<br />
289<br />
ALLEGRA<br />
ALLEGRA<br />
D<br />
Fexofenadine<br />
HCl<br />
Fexofenadine<br />
HCl<br />
Hoechst<br />
Marion<br />
Roussel<br />
Hoechst<br />
Marion<br />
Roussel<br />
Zoloft Sertraline Pfizer<br />
Roerig<br />
Imitrex<br />
DDAVP<br />
Sumatriptan<br />
Succinate<br />
Desmopressin<br />
Acetate<br />
Cerenex<br />
Glaxo<br />
Rhone<br />
Poulenc<br />
Rorer<br />
Accolate Zafirlukast Astra<br />
Zeneca<br />
CRIXIVAN<br />
Indinavir<br />
Sulfate<br />
Merck<br />
Co<br />
11/26/13<br />
5-578-610<br />
11/26/13<br />
5-578-610<br />
8/13/12<br />
5-249-699 08/07/00<br />
09/10/13<br />
5-554-639<br />
9/10/08<br />
5-407-398<br />
5-763-407<br />
01/06/13<br />
5-482-963<br />
05/07/13<br />
5-413-999<br />
ASACOL Mesalamine P&G 07/30/13<br />
5-541-171<br />
REZULIN Troglitazone Warner-L 09/13/13<br />
5-478-852<br />
Magnevist<br />
Viracept<br />
Gadapentetate<br />
Di-meglumine<br />
Nelfinavir<br />
Mesalate<br />
Berlex 10/01/13<br />
5-560-903<br />
Agguron 10/07/13<br />
5-484-926<br />
07/25/01 U-138<br />
NCE<br />
12/24/00 U-138<br />
NC<br />
8/26/00<br />
11/26/13<br />
11/26/13<br />
360<br />
420<br />
105<br />
140<br />
NCE 08/13/12 1629<br />
1780<br />
NDF<br />
U-72<br />
09/10/13 898<br />
990<br />
M/D/YY [09/10/08]<br />
12/23/13<br />
170<br />
210<br />
- - 01/09/13 146<br />
159<br />
3/13/01 U-132<br />
NCE<br />
8/18/00 U-141<br />
I-203<br />
01/29/02 U-163<br />
NCE<br />
- -<br />
-<br />
03/14/02 -<br />
NCE<br />
05/07/13 335<br />
356<br />
07/30/13 163<br />
183<br />
09/13/13 855<br />
930<br />
10/1/13 128<br />
155<br />
10/07/13 399<br />
410<br />
NA = Not Applicable ODE = Orphan Drug Exclusivity NS = New Strength<br />
NCE = New Chemical Entity NE = New Active Salt or Ester NP = New Drug Product<br />
NDF = New Dosage Form NC = New Combination 2001 1 USA + EU Sales<br />
U = Code for Therapeutic Use still I = Code for specific Indication D = New Dosing Schedule<br />
under Patent Protection<br />
under Exclusivity Protection<br />
Drugs Off-Patent Reports 22 Over USD$ 100 million