Soft Matter and Functional Materials - Helmholtz-Zentrum Berlin
Soft Matter and Functional Materials - Helmholtz-Zentrum Berlin
Soft Matter and Functional Materials - Helmholtz-Zentrum Berlin
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Research on <strong>Soft</strong> <strong>Matter</strong> ________________________________________________________<br />
Polymers <strong>and</strong> nanocomposites<br />
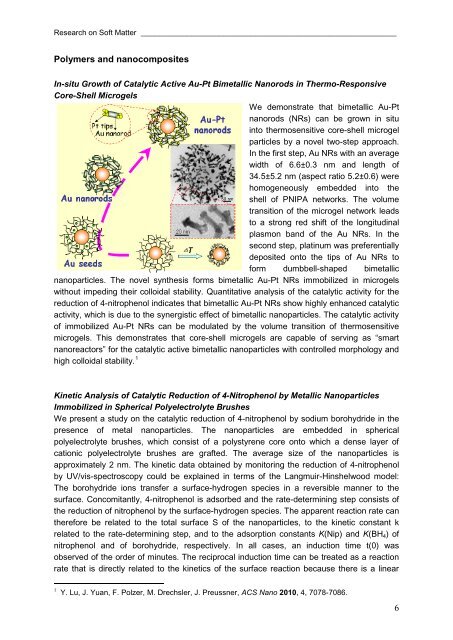
In-situ Growth of Catalytic Active Au-Pt Bimetallic Nanorods in Thermo-Responsive<br />
Core-Shell Microgels<br />
We demonstrate that bimetallic Au-Pt<br />
nanorods (NRs) can be grown in situ<br />
into thermosensitive core-shell microgel<br />
particles by a novel two-step approach.<br />
In the first step, Au NRs with an average<br />
width of 6.6±0.3 nm <strong>and</strong> length of<br />
34.5±5.2 nm (aspect ratio 5.2±0.6) were<br />
homogeneously embedded into the<br />
shell of PNIPA networks. The volume<br />
transition of the microgel network leads<br />
to a strong red shift of the longitudinal<br />
plasmon b<strong>and</strong> of the Au NRs. In the<br />
second step, platinum was preferentially<br />
deposited onto the tips of Au NRs to<br />
form dumbbell-shaped bimetallic<br />
nanoparticles. The novel synthesis forms bimetallic Au-Pt NRs immobilized in microgels<br />
without impeding their colloidal stability. Quantitative analysis of the catalytic activity for the<br />
reduction of 4-nitrophenol indicates that bimetallic Au-Pt NRs show highly enhanced catalytic<br />
activity, which is due to the synergistic effect of bimetallic nanoparticles. The catalytic activity<br />
of immobilized Au-Pt NRs can be modulated by the volume transition of thermosensitive<br />
microgels. This demonstrates that core-shell microgels are capable of serving as “smart<br />
nanoreactors” for the catalytic active bimetallic nanoparticles with controlled morphology <strong>and</strong><br />
high colloidal stability. 1<br />
Kinetic Analysis of Catalytic Reduction of 4-Nitrophenol by Metallic Nanoparticles<br />
Immobilized in Spherical Polyelectrolyte Brushes<br />
We present a study on the catalytic reduction of 4-nitrophenol by sodium borohydride in the<br />
presence of metal nanoparticles. The nanoparticles are embedded in spherical<br />
polyelectrolyte brushes, which consist of a polystyrene core onto which a dense layer of<br />
cationic polyelectrolyte brushes are grafted. The average size of the nanoparticles is<br />
approximately 2 nm. The kinetic data obtained by monitoring the reduction of 4-nitrophenol<br />
by UV/vis-spectroscopy could be explained in terms of the Langmuir-Hinshelwood model:<br />
The borohydride ions transfer a surface-hydrogen species in a reversible manner to the<br />
surface. Concomitantly, 4-nitrophenol is adsorbed <strong>and</strong> the rate-determining step consists of<br />
the reduction of nitrophenol by the surface-hydrogen species. The apparent reaction rate can<br />
therefore be related to the total surface S of the nanoparticles, to the kinetic constant k<br />
related to the rate-determining step, <strong>and</strong> to the adsorption constants K(Nip) <strong>and</strong> K(BH4) of<br />
nitrophenol <strong>and</strong> of borohydride, respectively. In all cases, an induction time t(0) was<br />
observed of the order of minutes. The reciprocal induction time can be treated as a reaction<br />
rate that is directly related to the kinetics of the surface reaction because there is a linear<br />
1 Y. Lu, J. Yuan, F. Polzer, M. Drechsler, J. Preussner, ACS Nano 2010, 4, 7078-7086.<br />
6