DPCA2-1
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Diabetes<br />
& Primary Care Australia<br />
Vol 2 No 1 2017<br />
The primary care diabetes journal for healthcare professionals in Australia<br />
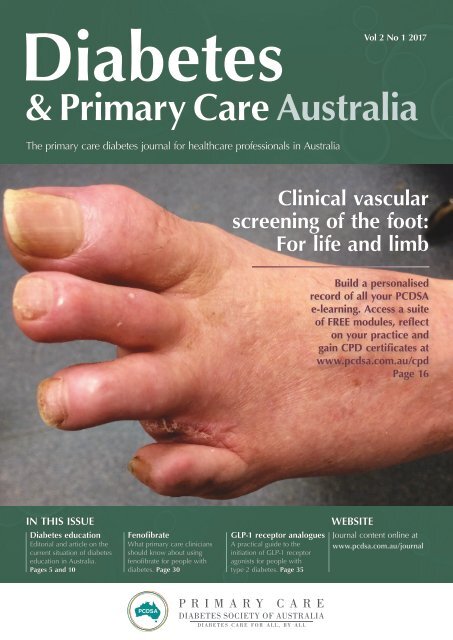
Clinical vascular<br />
screening of the foot:<br />
For life and limb<br />
Build a personalised<br />
record of all your PCDSA<br />
e-learning. Access a suite<br />
of FREE modules, reflect<br />
on your practice and<br />
gain CPD certificates at<br />
www.pcdsa.com.au/cpd<br />
Page 16<br />
IN THIS ISSUE<br />
Diabetes education<br />
Editorial and article on the<br />
current situation of diabetes<br />
education in Australia.<br />
Pages 5 and 10<br />
Fenofibrate<br />
What primary care clinicians<br />
should know about using<br />
fenofibrate for people with<br />
diabetes. Page 30<br />
GLP-1 receptor analogues<br />
A practical guide to the<br />
initiation of GLP-1 receptor<br />
agonists for people with<br />
type 2 diabetes. Page 35<br />
WEBSITE<br />
Journal content online at<br />
www.pcdsa.com.au/journal
The PCDSA is a multidisciplinary society with the aim<br />
of supporting primary health care professionals to deliver<br />
high quality, clinically effective care in order to improve<br />
the lives of people with diabetes.<br />
The PCDSA will<br />
Share best practice in delivering quality diabetes care.<br />
Provide high-quality education tailored to health professional needs.<br />
Promote and participate in high quality research in diabetes.<br />
Disseminate up-to-date, evidence-based information to health<br />
professionals.<br />
Form partnerships and collaborate with other diabetes related,<br />
high level professional organisations committed to the care of<br />
people with diabetes.<br />
Promote co-ordinated and timely interdisciplinary care.<br />
Membership of the PCDSA is free and members get access to a quarterly<br />
online journal and continuing professional development activities. Our first<br />
annual conference will feature internationally and nationally regarded experts<br />
in the field of diabetes.<br />
To register, visit our website:<br />
www.pcdsa.com.au
Contents<br />
Diabetes<br />
& Primary Care Australia<br />
Volume 2 No 1 2017<br />
Website: www.pcdsa.com.au/journal<br />
Editorial<br />
Diabetes education 5<br />
Rajna Ogrin reflects on the importance of diabetes education in Australia.<br />
From the desktop<br />
Patient and practitioner: Flash glucose monitoring 7<br />
Gary Kilov gives a first-hand perspective on using flash glucose monitoring.<br />
CPD module<br />
Clinical vascular screening of the foot: For life and limb 16<br />
Sylvia McAra, Robert Trevethan, Lexin Wang and Paul Tinley provide guidance to support early detection of peripheral arterial disease<br />
using evidence-based clinical tests.<br />
Articles<br />
Diabetes education: Essential but underfunded in Australia 10<br />
Mark Kennedy and Trisha Dunning explain why more is required to improve and provide diabetes education.<br />
Antimicrobial management of diabetic foot infection 25<br />
Roy Rasalam, Caroline McIntosh and Aonghus O’Loughlin provides an overview of the current evidence for diagnosis and<br />
management of diabetic foot infections in practice.<br />
What primary care clinicians should know about fenofibrate for people with diabetes 30<br />
Alicia J Jenkins, Andrzej S Januszewski, Emma S Scott and Anthony C Keech review fenofibrate's mechanisms of action,<br />
proven clinical benefits in type 2 diabetes and practical aspects of its prescription.<br />
GLP-1 receptor analogues – a practical guide to initiation 35<br />
Ralph Audehm and Laura Dean provide a practical guide to initiating glucagon-like peptide 1 analogues in people with type 2 diabetes.<br />
Editor-in-Chief<br />
Rajna Ogrin<br />
Senior Research Fellow, RDNS Institute, St Kilda, Vic<br />
Associate Editor<br />
Gary Kilov<br />
Practice Principal, The Seaport Practice, and Senior<br />
Lecturer, University of Tasmania, Launceston, Tas<br />
Editorial Board<br />
Ralph Audehm<br />
GP Director, Dianella Community Health, and<br />
Associate Professor, University of Melbourne,<br />
Melbourne, Vic<br />
Werner Bischof<br />
Periodontist, and Associate Professor, LaTrobe<br />
University, Bendigo, Vic<br />
Laura Dean<br />
Course Director of the Graduate Certificate in<br />
Pharmacy Practice, Monash University, Vic<br />
Nicholas Forgione<br />
Principal, Trigg Health Care Centre, Perth, WA<br />
John Furler<br />
Principal Research Fellow and Associate Professor,<br />
University of Melbourne, Vic<br />
Mark Kennedy<br />
Medical Director, Northern Bay Health, Geelong, and<br />
Honorary Clinical Associate Professor, University of<br />
Melbourne, Melbourne, Vic<br />
Peter Lazzarini<br />
Senior Research Fellow, Queensland University of<br />
Technology, Brisbane, Qld<br />
Roy Rasalam<br />
Head of Clinical Skills and Medical Director,<br />
James Cook University, and Clinical Researcher,<br />
Townsville Hospital, Townsville, Qld<br />
Suzane Ryan<br />
Practice Principal, Newcastle Family Practice,<br />
Newcastle, NSW<br />
Editor<br />
Olivia Tamburello<br />
Editorial Manager<br />
Richard Owen<br />
Publisher<br />
Simon Breed<br />
© OmniaMed SB and the Primary Care<br />
Diabetes Society of Australia<br />
Published by OmniaMed SB,<br />
1–2 Hatfields, London<br />
SE1 9PG, UK<br />
All rights reserved. No part of this<br />
journal may be reproduced or transmitted<br />
in any form, by any means, electronic<br />
or mechanic, including photocopying,<br />
recording or any information retrieval<br />
system, without the publisher’s<br />
permission.<br />
ISSN 2397-2254<br />
Diabetes & Primary Care Australia Vol 2 No 1 2017 3
Call for papers<br />
Would you like to write an article<br />
for Diabetes & Primary Care Australia?<br />
The new journal from the Primary Care Diabetes Society of Australia<br />
To submit an article or if you have any queries, please contact: gary.kilov@pcdsa.com.au.<br />
Title page<br />
Please include the article title, the full names of the authors<br />
and their institutional affiliations, as well as full details of<br />
each author’s current appointment. This page should also have<br />
the name, address and contact telephone number(s) of the<br />
corresponding author.<br />
Article points and key words<br />
Four or five sentences of 15–20 words that summarise the major<br />
themes of the article. Please also provide four or five key words<br />
that highlight the content of the article.<br />
Abstract<br />
Approximately 150 words briefly introducing your article,<br />
outlining the discussion points and main conclusions.<br />
Introduction<br />
In 60–120 words, this should aim to draw the reader into the<br />
article as well as broadly stating what the article is about.<br />
Main body<br />
Use sub-headings liberally and apply formatting to differentiate<br />
between heading levels (you may have up to three heading levels).<br />
The article must have a conclusion, which should be succinct and<br />
logically ordered, ideally identifying gaps in present knowledge and<br />
implications for practice, as well as suggesting future initiatives.<br />
Tables and illustrations<br />
Tables and figures – particularly photographs – are encouraged<br />
wherever appropriate. Figures and tables should be numbered<br />
consecutively in the order of their first citation in the text. Present<br />
tables at the end of the articles; supply figures as logically labelled<br />
separate files. If a figure or table has been published previously,<br />
acknowledge the original source and submit written permission<br />
from the copyright holder to reproduce the material.<br />
References<br />
In the text<br />
Use the name and year (Harvard) system for references in the<br />
text, as exemplified by the following:<br />
● As Smith and Jones (2013) have shown …<br />
● As already reported (Smith and Jones, 2013) …<br />
For three or more authors, give the first author’s surname<br />
followed by et al:<br />
● As Robson et al (2015) have shown …<br />
Simultaneous references should be ordered chronologically first,<br />
and then alphabetically:<br />
● (Smith and Jones, 2013; Young, 2013; Black, 2014).<br />
Statements based on a personal communication should be<br />
indicated as such, with the name of the person and the year.<br />
In the reference list<br />
The total number of references should not exceed 30 without prior<br />
discussion with the Editor. Arrange references alphabetically first,<br />
and then chronologically. Give the surnames and initials of all<br />
authors for references with four or fewer authors; for five or more,<br />
give the first three and add “et al”. Papers accepted but not yet<br />
published may be included in the reference list as being “[In press]”.<br />
Journal article example: Robson R, Seed J, Khan E et al (2015)<br />
Diabetes in childhood. Diabetes Journal 9: 119–23<br />
Whole book example: White F, Moore B (2014) Childhood<br />
Diabetes. Academic Press, Melbourne<br />
Book chapter example: Fisher M (2012) The role of age. In: Merson<br />
A, Kriek U (eds). Diabetes in Children. 2nd edn. Academic Press,<br />
Melbourne: 15–32<br />
Document on website example: Department of Health (2009)<br />
Australian type 2 diabetes risk assessment tool (AUSDRISK).<br />
Australian Government, Canberra. Available at: http://www.<br />
health.gov.au/preventionoftype2diabetes (accessed 22.07.15)<br />
Article types<br />
Articles may fall into the categories below. All articles should be<br />
1700–2300 words in length and written with consideration of<br />
the journal’s readership (general practitioners, practice nurses,<br />
prescribing advisers and other healthcare professionals with an<br />
interest in primary care diabetes).<br />
Clinical reviews should present a balanced consideration of a<br />
particular clinical area, covering the evidence that exists. The<br />
relevance to practice should be highlighted where appropriate.<br />
Original research articles should be presented with sections<br />
for the background, aims, methods, results, discussion and<br />
conclusion. The discussion should consider the implications<br />
for practice.<br />
Clinical guideline articles should appraise newly published<br />
clinical guidelines and assess how they will sit alongside<br />
existing guidelines and impact on the management of diabetes.<br />
Organisational articles could provide information on newly<br />
published organisational guidelines or explain how a particular<br />
local service has been organised to benefit people with diabetes.<br />
— Diabetes & Primary Care Australia —
Editorial<br />
Diabetes education<br />
We are all aware that diabetes<br />
mellitus prevalence is rising,<br />
affecting around half a billion<br />
people worldwide (International Diabetes<br />
Federation, 2015) and approximately 5%<br />
of Australians (917 000 people; Australian<br />
Institute of Health and Welfare, 2012).<br />
Unfortunately, almost half of people with<br />
type 2 diabetes have glycaemic levels out<br />
of target range (Si et al, 2010), leading to<br />
increased rates of macro- and microvascular<br />
complications and early mortality (Holman<br />
et al, 2008), as well as increasing healthcare<br />
costs (Lee et al, 2013).<br />
Diabetes education is pivotal in supporting<br />
effective diabetes self-management, and<br />
structured diabetes education supports selfmanagement,<br />
having been shown to improve<br />
blood glucose levels, blood pressure, weight<br />
and lipid levels, as well as having a positive<br />
effect on blood glucose self-monitoring<br />
(Norris et al, 2001; 2002; Ellis et al, 2004;<br />
Minet et al, 2010). Despite the advantages of<br />
structured education, over 40% of Australians<br />
with diabetes do not have access to such<br />
programs (Deloittes Access Economics, 2014).<br />
To provide an independent analysis of<br />
the cost effectiveness of diabetes education,<br />
the Australian Diabetes Educator Association<br />
(ADEA) commissioned Deloittes Access<br />
Economics (2014) to produce the report<br />
Benefits of Credentialled Diabetes Educators to<br />
people with diabetes and Australia. The report<br />
identified that for every $173 investment in<br />
diabetes education, there would be a return<br />
of $2827 per patient, per annum in healthcare<br />
cost savings. These cost savings were due<br />
to a reduction in frequency of hospital<br />
admission, emergency presentation, GP<br />
visits and treatment of related comorbidities.<br />
If diabetes education was made available<br />
to all Australians with diabetes, the total<br />
healthcare cost savings in 2014 would have<br />
been $3.9 billion and the lives of thousands<br />
of people with diabetes would have been<br />
improved.<br />
Unfortunately, many private health<br />
insurance companies do not fund diabetes<br />
education services, and current Medicare<br />
funding includes diabetes education as part<br />
of the five annual team care arrangement<br />
visits, alongside podiatry, dietetics and other<br />
associated healthcare providers. This means<br />
that many Australians with diabetes either<br />
have to pay for sessions with a diabetes<br />
educator themselves or miss out entirely. Outof-pocket<br />
costs for people with diabetes is<br />
one of the main barriers to accessing diabetes<br />
education (Deloittes Access Economics,<br />
2014).<br />
More is needed to support all Australians<br />
with diabetes accessing diabetes education.<br />
One way to do this is through private<br />
health insurers funding diabetes education.<br />
This would be feasible given that diabetes<br />
education is a relatively inexpensive cost<br />
compared to the healthcare costs incurred<br />
later on from sub-optimally managed diabetes<br />
(Deloittes Access Economics, 2014), such as<br />
the management of blindness, amputation,<br />
kidney failure and early mortality. Some<br />
therapies, which have less evidence for<br />
effectiveness compared to diabetes education,<br />
are currently reimbursed by private health<br />
insurance companies. Effective support of<br />
optimal diabetes management would lead<br />
to significant gains in the health of many<br />
people, as well as reduced health costs. Of<br />
course, private health insurance-funded<br />
diabetes education will only increase access<br />
to those who have private health insurance.<br />
Many people with the poorest diabetes<br />
outcomes are those of low socio-economic<br />
status and marginalised groups who are likely<br />
to not have private health insurance. In this<br />
issue, Mark Kennedy and Trisha Dunning<br />
provide the evidence for structured education<br />
and helpful guidance to encourage uptake<br />
(page 10). They highlight the importance of<br />
diabetes education in supporting people with<br />
diabetes to achieve optimal management of<br />
their diabetes while also highlighting the<br />
Rajna Ogrin<br />
Editor of Diabetes & Primary Care<br />
Australia, and Senior Research<br />
Fellow, RDNS Institute, St Kilda,<br />
Vic.<br />
Diabetes & Primary Care Australia Vol 2 No 1 2017 5
Editorial<br />
“Many people<br />
with the poorest<br />
diabetes outcomes<br />
are those of low<br />
socio-economic status<br />
and marginalised<br />
groups who are likely<br />
to not have private<br />
health insurance.”<br />
funding constraints that limit access for some<br />
people.<br />
Also in this issue<br />
This issue also includes a CPD module<br />
on peripheral arterial disease screening.<br />
Read the article on page 16 and then go<br />
to www.pcdsa.com.au/cpd to complete the<br />
10-question module. After completing the<br />
module you will receive a certificate that<br />
can go towards your continued professional<br />
development. There is an additional article<br />
on page 25 on antimicrobial infection of<br />
foot ulcers in people with diabetes by Roy<br />
Rasalam, Caroline McIntosh and Aonghus<br />
O’Loughlin.<br />
Ralph Audehm and Laura Dean provide<br />
a practical guidance on using and initiating<br />
glucagon-like peptide-1 analogues in people<br />
with type 2 diabetes (page 35), and there is<br />
an interesting, practical article on the role of a<br />
readily available, but under-used medication,<br />
fenofibrate, a cholesterol-lowering drug. This<br />
issue’s From the Desktop is by Gary Kilov,<br />
who provides the practitioner, and patient,<br />
perspective on flash glucose monitoring on<br />
page 7.<br />
n<br />
Australian Institute of Health and Welfare (2012) Diabetes.<br />
Australian Government, Canberra, ACT. Available at: http://<br />
www.aihw.gov.au/diabetes/prevalence/ (accessed 10.08.12)<br />
Deloittes Access Economics (2014) Benefits of Credentialled<br />
Diabetes Educators (CDEs) to people with diabetes in Australia.<br />
Australian Diabetes Educators Association, Canberra, Australia<br />
Ellis SE, Speroff T, Dittus RS et al (2004) Diabetes patient<br />
education: a meta-analysis and meta-regression. Patient Educ<br />
Couns 52: 97–105<br />
Holman RR, Paul SK, Bethel MA et al (2008) 10-Year follow-up of<br />
intensive glucose control in type 2 diabetes. New Engl J Med<br />
359: 1577–89<br />
IDF (2015) IDF Diabetes Atlas (7 th edition). International Diabetes<br />
Federation, Brussels, Belgium<br />
Lee CMY, Colagiuri R, Magliano DJ et al (2013) The cost of diabetes<br />
in adults in Australia. Diabetes Res Clin Pract 99: 385–90<br />
Minet L, Maller S, Vach W et al (2010) Mediating the effect of<br />
self-care management intervention in type 2 diabetes: A metaanalysis<br />
of 47 randomised controlled trials. Patient Educ Couns<br />
80: 24–41<br />
Norris SL, Engelgau MM, Narayan KMV (2001) Effectiveness of selfmanagement<br />
training in type 2 diabetes: a systematic review of<br />
randomized controlled trials. Diabetes Care 24: 561–87<br />
Norris SL, Lau J, Smith SJ et al (2002) Self-management education<br />
for adults with type 2 diabetes: a meta-analysis of the effect on<br />
glycemic control. Diabetes Care 25: 1159–71<br />
Si D, Bailie R, Wang Z, Weeramanthri T (2010) Comparison<br />
of diabetes management in five countries for general and<br />
indigenous populations: an internet-based review. BMC Health<br />
Services Research 10: 169<br />
6 Diabetes & Primary Care Australia Vol 2 No 1 2017
From the desktop<br />
From the desktop<br />
Patient and practitioner:<br />
Flash glucose monitoring<br />
Gary Kilov<br />
I<br />
have been caring for people with diabetes<br />
for about three decades, which is similar to<br />
the length of time that I have been living<br />
with type 1 diabetes. It would, therefore, come<br />
as no surprise that I have a vested interest in<br />
keeping abreast of the latest developments and<br />
innovations as they pertain to the management<br />
of diabetes – specifically, any progress that<br />
improves quality of life and eases the burden<br />
of living with diabetes. When I am fortunate<br />
enough to be offered the opportunity to try<br />
out some of the more innovative new products,<br />
I jump at the opportunity, and such was the<br />
case with the FreeStyle Libre Flash Glucose<br />
Monitoring System (Abbott Diabetes Care,<br />
Alameda, California, USA).<br />
Among the most significant advances in<br />
diabetes management in recent decades has<br />
been the progress in glucose sensing. For over<br />
2000 years, urine tasters were trained to detect<br />
glycosuria (Kirchoff et al, 2008); that is, until the<br />
the first half of the 20 th century, when chemical<br />
reagents took the place of taste buds to detect<br />
glucose in urine. Since then, the pace of change<br />
has been rapid, progressing from testing urine<br />
with tablets or strips to measuring glucose in<br />
blood. Regular, frequent blood glucose level<br />
(BGL) testing is essential for patients on multiple<br />
doses of insulin to guide dosing and optimise<br />
glycaemic management whilst mitigating<br />
hypoglycaemia. Until recently, the gold standard<br />
for BGL sensing for most of our patients has<br />
been self-blood glucose monitoring using fingerprick<br />
testing. Despite significant improvements<br />
in this technology, several limitations remain.<br />
Finger-prick testing is inconvenient, painful and<br />
gives only a momentary snapshot in time, falling<br />
short of providing a comprehensive profile of<br />
dynamically changing BGLs. Ideally, continuous<br />
monitoring of BGLs should be more widely<br />
available for people with diabetes as emerging<br />
data supports its effectiveness in maintaining<br />
glycaemic control, and international professional<br />
organisations endorse it as the gold standard for<br />
those with type 1 diabetes (Endocrine Society,<br />
2016).<br />
Since the turn of this century, access to<br />
continuous glucose monitoring (CGM) has been<br />
steadily increasing but significant limitations and<br />
barriers remain. It is more costly than traditional<br />
blood glucose monitoring and is, therefore,<br />
limited to a small cohort of patients, usually<br />
those with type 1 diabetes using insulin pumps<br />
and to whom the cost has not been a barrier.<br />
Over time, the affordability of CGM devices<br />
has improved and the entry of new players into<br />
the space has resulted in gradual, but steadily<br />
increasing access.<br />
However, it is only with the release<br />
of the FreeStyle Libre that we have seen a<br />
democratisation of this space with the device<br />
promoted to healthcare providers, but firmly<br />
marketed directly to consumers who have driven<br />
the demand. As clichéd as this may sound, this<br />
has been a game changer. The FreeStyle Libre<br />
provides greater accessibility for consumers and,<br />
not surprisingly, has proven very popular. The<br />
system has been available in the UK and Europe<br />
for almost 3 years, while in Australia it has been<br />
available for several months. By all accounts so<br />
far, it has proven to be just as popular here as in<br />
the northern hemisphere.<br />
So what does it do? The FreeStyle Libre offers<br />
flash glucose sensing. What this entails is the<br />
application of a sensor (which lasts for 2 weeks<br />
before requiring a replacement) to the upper arm<br />
Citation: Kilov G (2017) Patient<br />
and practitioner: Flash glucose<br />
monitoring. Diabetes & Primary Care<br />
Australia 2: 7–9<br />
About this series<br />
The aim of the “From the desktop”<br />
series is to provide practical<br />
expert opinion and comment<br />
from the clinic. In this issue, Gary<br />
Kilov gives a first-hand patient and<br />
practitioner perspective on using<br />
flash glucose monitoring.<br />
Author<br />
Gary Kilov is Associate Editor of<br />
Diabetes & Primary Care Australia,<br />
and Director at Seaport Diabetes,<br />
Launceston Area, Tas, and Senior<br />
Lecturer at University of Tasmania,<br />
Launceston, Tas.<br />
Diabetes & Primary Care Australia Vol 2 No 1 2017 7
From the desktop<br />
“On the infrequent<br />
occasion that I have to<br />
do a finger-prick test,<br />
it reminds me how<br />
much I don’t miss it.”<br />
Figure 1. The FreeStyle Libre Flash Glucose Monitoring System (Abbott, Diabetes Care, Alameda, California, USA),<br />
and the sensor and reader in use.<br />
and a reader that, when waved over the sensor,<br />
connects wirelessly and downloads and displays<br />
the BGL readings. As long as the reader is waved<br />
over the sensor at least once every 8 hours, up to<br />
8 hours of stored information will be transferred<br />
to the reader and will be instantly displayed.<br />
This includes the current BGL and a graph of<br />
the day’s glucose readings, as well as a display<br />
of trend arrows indicating whether the BGL<br />
is rising or falling. The angle of the depicted<br />
arrows indicates whether the BGL is trending<br />
higher or lower, rapidly or gradually, or is in<br />
fact steady.<br />
Whilst this information is available with<br />
CGM, flash glucose monitoring is different in<br />
that no calibration is required using finger-prick<br />
testing. This feature is particularly attractive to<br />
me, and dare I say, to all my patients using this<br />
device. On the infrequent occasion that I have<br />
to do a finger-prick test, it reminds me how<br />
much I don’t miss it. There are times when it<br />
is wise to confirm BGL reading with a fingerprick<br />
test and this is detailed in the product<br />
information and borne out by real-world<br />
experience. When BGLs are trending rapidly,<br />
the lag in interstitial fluid glucose means that<br />
the accuracy of the result is compromised.<br />
This has been particularly important when my<br />
BGLs are trending down. I am also prompted<br />
occasionally to do a finger-prick test when I<br />
am experiencing symptoms that are discordant<br />
with the readings, irrespective of what the<br />
BGL readings or trend arrows might indicate.<br />
So, in general, how accurate do I find the<br />
FreeStyle Libre? I can say that, by and large, I<br />
have discontinued finger-prick testing and use<br />
it only occasionally as detailed above, as flash<br />
monitoring has proven to be very reliable.<br />
Another attraction of this system for me<br />
has been the deeper understanding that I have<br />
gained. The FreeStyle Libre has afforded me<br />
a greater degree of finesse in managing my<br />
diabetes. Whilst this may be a honeymoon<br />
phase with my new-found love, the Libre, my<br />
HbA 1c<br />
has dropped by 0.5% (5.5 mmol/mol),<br />
from already low levels, without an increase<br />
in hypoglycaemia. I’ve also developed some<br />
insights into what happens to my BGLs in<br />
certain situations that would have previously<br />
been difficult to discern. A case in point is<br />
real-time BGL monitoring during exercise. This<br />
has allowed me to manage my glycaemia more<br />
confidently by understanding my blood glucose<br />
patterns in response to certain stimuli and,<br />
therefore, anticipate my BGL trajectory and the<br />
appropriate proactive interventions to take. For<br />
example, as readers would know, BGLs tend to<br />
rise with exercise followed by the potential risk<br />
for delayed hypoglycaemia, as muscles replenish<br />
their spent stores of glycogen, and increased postexercise<br />
insulin sensitivity. What I discovered<br />
by careful experimentation was that by giving<br />
myself just one unit of rapid-acting insulin<br />
15 minutes before I exercise (something I would<br />
never have been comfortable doing prior to<br />
having the FreeStyle Libre) I have been able to<br />
mitigate the exercise-induced hyperglycaemia.<br />
By simply removing that one unit from the next<br />
8 Diabetes & Primary Care Australia Vol 2 No 1 2017
From the desktop<br />
dose, I have also been able to reduce the rate of<br />
post-exercise hypoglycaemia.<br />
And what of patient experience? For users<br />
of flash monitoring, this has generally been<br />
very positive. Sensor failure or a sensor failing<br />
to adhere occurred rarely in the early days.<br />
This has been easily corrected by improving<br />
the application technique of the sensor, and<br />
there have been no subsequent sensor failures<br />
or loss of sensors reported. I now make a point<br />
of inviting patients to see me or the practice’s<br />
diabetes educator for the initial application of<br />
the sensor in a bid to obviate potential errors<br />
with the system. The FreeStyle Libre can also<br />
be a boon for “significant others” who may fear<br />
undetected nocturnal hypos. My wife need only<br />
“flash” my sensor for a quick check of my BGLs<br />
and decide, with confidence, whether to wake<br />
me to treat a hypo or allow me to slumber on. A<br />
win–win situation.<br />
However, sadly, nothing is perfect. So<br />
what are the downsides? Whilst the system<br />
is a great improvement on self-monitoring of<br />
BGLs, it is not without room for improvement.<br />
Cost remains prohibitive for some. At $100<br />
per fortnight on an ongoing basis, this is<br />
unaffordable for many. One compromise is to<br />
use the FreeStyle Libre much as we currently use<br />
CGM – using a sensor intermittently to provide<br />
insights and make adjustments as necessary.<br />
When a sensor is not in use, the reader can<br />
be used as a standalone BGL meter that will<br />
measure both BGLs and ketones using the same<br />
strips used in the FreeStyle Optium Neo.<br />
One of the great strengths of the system<br />
is the enormous amount of information that<br />
it provides. Paradoxically, for some, this is<br />
a disadvantage. Having lots of data at one’s<br />
disposal is one thing, what to do with it is<br />
another. It can also be quite difficult for some<br />
patients, particularly those who like to micromanage,<br />
to curtail the urge to react to every<br />
trend or nuanced change highlighted by the<br />
Libre. Additionally, there is no low glucose<br />
alarm on the Libre, a feature present in CGMs.<br />
Even though low BGLs may be recorded by the<br />
Libre sensor, no alerts are sounded until the<br />
sensor is scanned.<br />
What’s on the wish list for glucose monitoring?<br />
It has recently been announced that there will<br />
be CGM subsidies for children and young<br />
people with type 1 diabetes, and it is on my<br />
wish list for subsidies to be available for adults,<br />
as well as for flash glucose monitoring to be<br />
covered in addition to CGM. If CGM or flash<br />
glucose monitoring were more affordable, it<br />
would unquestionably be the system of choice<br />
for both individuals with type 1 diabetes and<br />
those with type 2 diabetes on complex insulin<br />
regimens.<br />
Overall, the FreeStyle Libre has been a boon<br />
for me and many other people with diabetes<br />
who have used flash glucose monitoring. It has<br />
improved our quality of life as well as improved<br />
our ability to manage our diabetes, and I<br />
am looking forward to the next innovation.<br />
Perhaps I could give the bionic pancreas a<br />
test drive…?<br />
n<br />
Declaration<br />
Selected healthcare professionals, especially<br />
endocrinologists, diabetes educators, and<br />
GPs specialising in diabetes management,<br />
were offered the opportunity to participate in<br />
the FreeStyle Libre Healthcare Professional<br />
Experience Program. A FreeStyle Libre Reader<br />
and two FreeStyle Libre Sensors were provided,<br />
free of charge to Gary Kilov, as part of the<br />
FreeStyle Libre HCP Experience Program. All<br />
subsequent sensors used by Gary Kilov have been<br />
purchased from the Australian Freestyle Libre<br />
website as per all consumers. No inducements,<br />
honoraria or support was provided to write<br />
this comment, which is an independent and<br />
personal reflection on the Flash Libre and its<br />
utility.<br />
Endocrine Society (2016) Experts recommend continuous<br />
glucose monitors for adults with type 1 diabetes.<br />
Endocrine Society, Washington DC, USA. Available at:<br />
http://bit.ly/2i4MKeT (accessed 30.11.16)<br />
Kirchhof M, Popat N, Malowany J (2008) A Historical Perspective<br />
of the Diagnosis of Diabetes. UWOMJ 70: 7–11<br />
“If continuous<br />
glucose monitoring<br />
or flash glucose<br />
monitoring were more<br />
affordable, it would<br />
unquestionably be<br />
the system of choice<br />
for both individuals<br />
with type 1 diabetes<br />
and those with type 2<br />
diabetes on complex<br />
insulin regimens.”<br />
Diabetes & Primary Care Australia Vol 2 No 1 2017 9
Article<br />
Diabetes education: Essential but<br />
underfunded in Australia<br />
Citation: Kennedy M, Dunning T<br />
(2017) Diabetes education: Essential<br />
but underfunded in Australia.<br />
Diabetes & Primary Care Australia<br />
2: 10–4<br />
Article points<br />
1. More work is needed to<br />
support improved access for<br />
Australians with diabetes to<br />
education, and thereby achieve<br />
optimal glycaemic levels and<br />
reduce early mortality and<br />
complication development.<br />
2. Despite a multitude of new<br />
treatments for lowering<br />
cardiovascular risk factors,<br />
many people with diabetes<br />
remain far above target levels.<br />
3. Structured diabetes education<br />
has beneficial effects<br />
on blood glucose, lipids<br />
and blood pressure and<br />
specialist attendance rates.<br />
4. Structured diabetes education<br />
remains underfunded by<br />
Government and private<br />
health insurers, and this<br />
restricts access for people<br />
with diabetes contributing to<br />
sub-optimal health outcomes.<br />
Key words<br />
– Access to education<br />
– Diabetes educator<br />
– Health funding<br />
Authors<br />
Mark Kennedy is Honorary<br />
Clinical Associate Professor,<br />
Department of General Practice,<br />
University of Melbourne, and a<br />
GP, Geelong, Vic. Trisha Dunning<br />
is Chair in Nursing and Director<br />
for the Centre for Nursing and<br />
Allied Health Research, Deakin<br />
University and Barwon Health,<br />
Melbourne, Vic.<br />
Mark Kennedy, Trisha Dunning<br />
Many people with diabetes develop comorbidities during their lives. These include<br />
diabetes-related complications, such as cardiovascular disease, neuropathy and chronic<br />
kidney disease, and other medical problems such as arthritis, heart failure and depression.<br />
These complications and comorbidities can adversely affect mental health and self-care,<br />
and contribute to premature decline in functional status, morbidity, mortality and a<br />
significant reduction in quality of life. Despite better understanding of the natural history<br />
of diabetes and a multitude of new treatments for lowering the risk factors of the disease,<br />
many people with diabetes remain far above target levels. Structured diabetes education<br />
has beneficial effects on blood glucose, lipids and blood pressure and specialist attendance<br />
rates. Structured diabetes education remains underfunded by the Australian Government<br />
and private health insurers. Given the growing rates of diabetes and earlier diagnosis of<br />
the disease, without increased access to diabetes education for Australians with diabetes,<br />
suboptimal health outcomes will continue. Australia needs to do more to make diabetes<br />
education accessible to all people with type 2 diabetes.<br />
Diabetes mellitus is a complex, chronic<br />
and progressive disease affecting<br />
multiple body organs and systems. The<br />
prevalence in Australia in 2015 was estimated<br />
to be 6.3% of adults, representing more than<br />
1 million adults, with almost another 500 000<br />
thought to meet criteria for a diagnosis of<br />
diabetes but who are still undiagnosed<br />
(International Diabetes Federation [IDF]<br />
Diabetes Atlas Committee, 2015). It has also<br />
been estimated that the mean annual diabetesrelated<br />
expenditure per person with diabetes<br />
in Australia was more than $7600 in 2015,<br />
and that there were more than 6300 diabetesrelated<br />
deaths in Australia in the same year<br />
(IDF Diabetes Atlas Committee, 2015). The<br />
prevalence of diabetes has been rising across the<br />
world for many years and, in recent decades,<br />
there has been a fall in the average age of onset,<br />
so the number of cases on type 2 diabetes in<br />
the young has been rising (Alberti et al, 2004).<br />
With earlier onset type 2 diabetes, the increased<br />
lifetime exposure to hyperglycaemia is associated<br />
with a higher complication rate over time<br />
(Constantino et al, 2013). Many of these people<br />
have or will develop comorbidities during their<br />
lives, including diabetes-related complications<br />
and conditions, such as cardiovascular disease,<br />
neuropathy and chronic kidney disease, and<br />
other medical problems such as arthritis, heart<br />
failure and depression (Haas et al, 2013).<br />
These complications and comorbidities can<br />
adversely affect mental health and self-care, and<br />
contribute to premature decline in functional<br />
status, morbidity, mortality and significantly<br />
reduce quality of life (UK Prospective Diabetes<br />
Study Group, 1999; Skovlund and Peyrot,<br />
2005; Huxley et al, 2006; Seshasai et al, 2011).<br />
Self-care is often made more difficult by the<br />
emotional toll associated with the diagnosis of<br />
diabetes, the progressive nature of the condition<br />
and the emotional toll from the need for<br />
constant attention and care (Peyrot et al, 2009).<br />
In recent years, there have been significant<br />
10 Diabetes & Primary Care Australia Vol 2 No 1 2017
Diabetes education: Essential but underfunded in Australia<br />
developments in the understanding and<br />
management of diabetes, and in the many<br />
ways in which complications of diabetes can<br />
be delayed or prevented. However, suboptimal<br />
management of many of the contributing<br />
factors to these complications, such as<br />
unhealthy lifestyle and elevated blood glucose,<br />
blood lipids and blood pressure, remains a<br />
significant problem for people with diabetes<br />
and the health care system. In all these areas,<br />
the most important person to address and<br />
optimally manage these factors is the person<br />
with diabetes. The person with diabetes needs<br />
timely and appropriate diabetes education to<br />
enable them to manage their diabetes.<br />
Diabetes education<br />
One of the goals of diabetes education<br />
is to assist people with diabetes to better<br />
understand their diabetes in order to enable<br />
them to make informed choices about selfmanagement,<br />
to improve their quality of<br />
life and to reduce the risk of complications<br />
(Australian Diabetes Educators Association,<br />
2016). This also increases the confidence of<br />
people with diabetes to manage their condition<br />
and assists them to undertake the practical<br />
aspects of monitoring and managing therapy<br />
(Australian Diabetes Educators Association,<br />
2016). Diabetes education also helps people<br />
with diabetes and their families to deal with<br />
the daily physical and emotional demands<br />
of the condition in the context of their<br />
social, cultural and economic circumstances<br />
(Australian Diabetes Educators Association,<br />
2016).<br />
Optimal diabetes management involves<br />
co-ordinated multidisciplinary care in the<br />
hospital setting and in the community,<br />
with the person with diabetes having the<br />
central role (Haas et al, 2013). Initial and<br />
ongoing education about diabetes is a critical<br />
process provided by all those involved in the<br />
multidisciplinary care of people with diabetes.<br />
This article focuses on the roles provided by<br />
diabetes educators and credentialled diabetes<br />
educators in Australia, and on the evidence<br />
supporting those roles, and highlights issues<br />
with access to these health professionals.<br />
Evidence for the importance and effectiveness<br />
of structured diabetes education<br />
There is randomised controlled trial evidence that<br />
structured diabetes education improves blood<br />
glucose levels, blood pressure, weight and lipid<br />
levels, as well as blood glucose self-monitoring<br />
(Norris et al, 2001; 2002; Ellis et al, 2004;<br />
Minet et al, 2010). There is also evidence that<br />
diabetes education can increase use of glucose,<br />
lipid and blood pressure-lowering medications,<br />
and consultation rates with optometrists or<br />
ophthalmologists (Murray and Shah, 2016).<br />
An Australian study showed that a structured<br />
education and treatment program can result in<br />
reduced mortality and reduced use of hospital<br />
services by people with type 2 diabetes (Lowe et<br />
al, 2009).<br />
While more recent local data is not available,<br />
the American Diabetes Association (ADA, 2013)<br />
estimated that in 2012 the average number<br />
of workdays lost per patient per year from<br />
diabetes was 1.1 days, with another 5.1 days<br />
characterised by reduced work performance<br />
and 5.8 days of reduced participation in the<br />
labour force. In 2002, it was estimated that<br />
the average income lost by patients and carers<br />
in Australia from type 2 diabetes was $35 per<br />
person per year, while income loss was higher<br />
when complications were present. However, the<br />
study population had a mean age of 65 years,<br />
so employment rates reflected that older age<br />
demographic, and the analysis did not include<br />
people with type 1 diabetes (Colagiuri, 2003).<br />
These analyses highlight the links between<br />
diabetes and reduced productivity, but studies<br />
connecting the provision of structured diabetes<br />
education to improvements in productivity have<br />
not yet been done.<br />
While the benefits of structured diabetes<br />
education are now well-established, there can be<br />
considerable variability in the amount and type<br />
of education provided. Comparison of studies<br />
examining effectiveness of diabetes education are<br />
often complicated by the variation in number<br />
of sessions provided, how they are delivered and<br />
the period of follow-up after study completion.<br />
Studies also vary in the methodology utilised for<br />
comparisons and in how rates of people dropping<br />
out of the study are managed. A recent meta-<br />
Page points<br />
1. One of the goals of diabetes<br />
education is to assist people<br />
with diabetes to better<br />
understand their diabetes in<br />
order to enable them to make<br />
informed choices about selfmanagement,<br />
to improve their<br />
quality of life and to reduce the<br />
risk of complications.<br />
2. An Australian study showed<br />
that a structured education and<br />
treatment program can result in<br />
reduced mortality and reduced<br />
use of hospital services by<br />
people with type 2 diabetes.<br />
3. While the benefits of structured<br />
diabetes education are now<br />
well-established, there is<br />
considerable variability in the<br />
amount and type of education<br />
provided.<br />
Diabetes & Primary Care Australia Vol 2 No 1 2017 11
Diabetes education: Essential but underfunded in Australia<br />
Page points<br />
1. A diabetes education<br />
assessment encompasses a<br />
detailed medical history, health<br />
beliefs and attitudes, baseline<br />
diabetes knowledge, cultural<br />
context, self-management skills,<br />
readiness to learn, general and<br />
health literacy, family and social<br />
support, and financial status.<br />
2. Effective diabetes education<br />
needs to be an ongoing process,<br />
involving ongoing support and<br />
reinforcement by the diabetes<br />
educator and other members of<br />
the multidisciplinary team.<br />
analysis showed that for every 1 hour of diabetes<br />
education provided, HbA 1c<br />
fell by an additional<br />
0.04% up to 28 hours of education, an amount<br />
that can be equal to the benefit provided by<br />
some glucose-lowering medications (Norris et<br />
al, 2002).<br />
Effective diabetes education requires teaching<br />
and assessment skills and the ability to<br />
personalise the information to the needs of the<br />
individual with diabetes (Australian Diabetes<br />
Educators Association, 2016). The increased<br />
benefits of individualised diabetes education<br />
are well-established and provide the basis of the<br />
initial assessment of a person with diabetes by a<br />
diabetes educator (Davis et al, 1981; Gilden et<br />
al, 1989; Davis et al, 1990; Glasgow et al, 1992;<br />
Brown, 1999). A diabetes education assessment<br />
encompasses a detailed medical history,<br />
health beliefs and attitudes, baseline diabetes<br />
knowledge, cultural context, self-management<br />
skills, readiness to learn, general and health<br />
literacy, family and social support, and financial<br />
status (Haas et al, 2013). An appropriate<br />
structured diabetes education program can be<br />
developed with and for the person with diabetes<br />
based on this assessment and using the various<br />
components of a diabetes educator’s role (See<br />
Table 1).<br />
Initial improvements in metabolic outcomes<br />
and other parameters after diabetes education<br />
often diminish over time, even after only 6 months<br />
(Norris et al, 2002). Effective diabetes education,<br />
therefore, needs to be an ongoing process<br />
involving ongoing support and reinforcement<br />
by the diabetes educator and other members<br />
of the multidisciplinary team. How often,<br />
and how intensive, the support and education<br />
is required will vary among individuals and<br />
Table 1. Core components of the diabetes educator role.*<br />
Role component Clinical input Competency<br />
Research<br />
Clinical practice<br />
Diabetes education<br />
• Translate research into practice and evaluate<br />
outcomes, and undertake audits and evaluate<br />
their practice.<br />
• Educate and support clinical staff to<br />
understand and use research.<br />
• Collaborate in or lead research.<br />
• Comprehensive clinical and educational<br />
assessment as part of the annual cycle of care.<br />
• Plan relevant care (personalised) and<br />
education with the individual with diabetes<br />
and often their families.<br />
• Deliver clinical care, such as foot care and<br />
wound care.<br />
• Provide diabetes education to individuals,<br />
groups and sometimes in public forums.<br />
• Provide diabetes education to care facility<br />
staff in undergraduate and postgraduate health<br />
professional education (e.g. nurses, allied<br />
health and medical students).<br />
• Supervise clinical placements.<br />
• Be able to read and analyse research reports<br />
and make decisions about the relevance to<br />
practice.<br />
• Understand glucose homeostasis and the<br />
impact of diabetes and related conditions.<br />
• Have an understanding of teaching and learning<br />
process.<br />
Management<br />
• Manage issues, such as referrals, product<br />
supply and clinical governance.<br />
• Facilitate complex communication pathways<br />
involving those with diabetes, their families,<br />
the other members of the multidisciplinary<br />
team, and one or more institutions involved in<br />
provision of care.<br />
• Staff management.<br />
• Collaborate with the interdisciplinary heath<br />
care team.<br />
• Help people with diabetes navigate transitions<br />
among services.<br />
• Have a solid understanding of good governance<br />
and service delivery systems.<br />
*The time spent in each component of the role depends on where the diabetes educator works and their position description.<br />
12 Diabetes & Primary Care Australia Vol 2 No 1 2017
Diabetes education: Essential but underfunded in Australia<br />
throughout their life course with diabetes. The<br />
effectiveness of diabetes education is enhanced<br />
when communication between multidisciplinary<br />
team members facilitates reinforcement of shared<br />
advice and when team members have agreed<br />
priorities with the people with diabetes they<br />
manage (Haas et al, 2013).<br />
Accessibility of diabetes education in Australia<br />
Despite the evidence supporting structured<br />
diabetes education, over 40% of Australians with<br />
diabetes do not have access to diabetes education<br />
programs (Deloittes Access Economics, 2014).<br />
Medicare funding through chronic disease<br />
management funding is often inadequate for the<br />
amount of initial and ongoing education required<br />
(Deloittes Access Economics, 2014), where a<br />
maximum of five individual sessions across all<br />
allied health staff per year is funded. The need for<br />
more education may be greater for those newly<br />
diagnosed or those transitioning onto injectable<br />
therapies and when functional status changes or<br />
complications develop. Many Australian private<br />
health insurance companies provide little or no<br />
coverage for diabetes education.<br />
Recently, the Australian Diabetes Educators<br />
Association commissioned Deloittes Access<br />
Economics to determine the cost-effectiveness<br />
of diabetes education in Australia. The report<br />
indicated that over $16 can be saved in health<br />
system costs for every dollar spent on diabetes<br />
education (Deloittes Access Economics,<br />
2014). The reduced spending results from a<br />
combination of fewer hospital admissions,<br />
emergency department attendances and physician<br />
consultations and the reduced costs from<br />
delayed or avoided secondary complications,<br />
such as retinopathy, chronic kidney disease,<br />
amputations, coronary heart disease and stroke.<br />
As the burden of diabetes on Australian<br />
families, our health care system and our<br />
economy continues to increase, providing<br />
adequate funding to train the additional required<br />
diabetes educator workforce and to ensure that<br />
all Australians with diabetes are able to access<br />
adequate structured diabetes education alongside<br />
their other multidisciplinary care must be a<br />
priority. Government and private health insurers<br />
must work together to address this underfunding<br />
of a vital component of diabetes care in this<br />
country.<br />
Conclusion<br />
Diabetes education is central to effective diabetes<br />
self-care to improve the ability of people with<br />
diabetes to self-manage, and has significant cost<br />
benefits and other benefits for the health system<br />
and individuals with diabetes. However the<br />
current funding for five allied health visits, which<br />
includes visits to diabetes education, is inadequate<br />
to meet those needs and is not consistent with<br />
the changes in information needs people with<br />
diabetes encounter over their life journey with<br />
diabetes. The lack of private health insurance<br />
funding of diabetes education contributes to the<br />
limited ability of people with diabetes to improve<br />
glycaemic self-management. More work is<br />
needed to support improved access of Australians<br />
with diabetes to diabetes education, and thereby<br />
achieve optimal glycaemic levels and reduce early<br />
mortality and complication development. n<br />
<br />
Alberti G, Zimmet P, Shaw et al (2004) Type 2 diabetes in the young:<br />
The evolving epidemic. The International Diabetes Federation<br />
Consensus Workshop. Diabetes Care 27: 1798–811<br />
American Diabetes Association (2013) Economic costs of diabetes in<br />
the U.S. in 2012. Diabetes Care 36: 1033–46<br />
Australian Diabetes Educators Association (2016) Diabetes selfmanagement<br />
education and credentialled diabetes educators<br />
[Online]. Australian Diabetes Educators Association, Woden,<br />
ACT. Available at: https://www.adea.com.au/about-us/ourpeople/diabetes-self-management-education-and-credentialleddiabetes-educators/<br />
(accessed 29.11.16)<br />
Brown SA (1999) Interventions to Promote Diabetes Self-<br />
Management: State of the Science. Diabetes Educator 25: 52–61<br />
Colagiuri SC, Conway B, Grainger D, Davy P (2003) DiabCo$t<br />
Australia: assessing the burden of type 2 diabetes in Australia.<br />
Diabetes Australia, Canberra Australia<br />
Constantino MI, Molyneaux L, Limacher-Gisler F et al (2013) Longterm<br />
complications and mortality in young-onset diabetes: type 2<br />
diabetes is more hazardous and lethal than type 1 diabetes.<br />
Diabetes Care 36: 3863–9<br />
Davis WK, Hull AL, Boutaugh ML (1981) Factors affecting the<br />
educational diagnosis of diabetic patients. Diabetes Care 4: 275<br />
Davis TC, Crouch MA, Wills G et al (1990) The gap between patient<br />
reading comprehension and the readability of patient education<br />
materials. Quadrant Healthcom Inc.<br />
Page points<br />
1. Despite the evidence<br />
supporting structured diabetes<br />
education, over 40% of<br />
Australians with diabetes do<br />
not have access to diabetes<br />
education programs.<br />
2. Providing adequate funding<br />
to train the additional<br />
required diabetes educator<br />
workforce and to ensure that<br />
all Australians with diabetes<br />
are able to access adequate<br />
structured diabetes education<br />
must be a priority.<br />
3. Diabetes education is central<br />
to effective diabetes self-care<br />
in order to improve the ability<br />
of people with diabetes to<br />
self-manage, and has significant<br />
cost benefits and other benefits<br />
for the health system and<br />
individuals with diabetes.<br />
Diabetes & Primary Care Australia Vol 2 No 1 2017 13
Diabetes education: Essential but underfunded in Australia<br />
Deloittes Access Economics (2014) Benefits of Credentialled<br />
Diabetes Educators (CDEs) to people with diabetes in Australia.<br />
Australian Diabetes Educators Association, Canberra, ACT<br />
Ellis SE, Speroff T, Dittus RS et al (2004) Diabetes patient education:<br />
a meta-analysis and meta-regression. Patient Educ Couns 52:<br />
97–105<br />
Minet L, Maller S, Vach W et al (2010) Mediating the effect of<br />
self-care management intervention in type 2 diabetes: A metaanalysis<br />
of 47 randomised controlled trials. Patient Educ Counsel<br />
80: 29–41<br />
Murray CM, Shah BR (2016) Diabetes self-management education<br />
improves medication utilization and retinopathy screening in the<br />
elderly. Primary Care Diabetes 10: 179–85<br />
Gilden JL, Hendryx M, Casia C, Singh SP (1989) The effectiveness of<br />
diabetes education programs for older patients and their spouses.<br />
J Amer Geriatrics Soc 37: 1023–30<br />
Norris SL, Engelgau MM, Narayan KM (2001) Effectiveness of selfmanagement<br />
training in type 2 diabetes: a systematic review of<br />
randomized controlled trials. Diabetes Care 24: 561–87<br />
Glasgow RE, Toobert DJ, Hampson SE et al (1992) Improving selfcare<br />
among older patients with type II diabetes: the ‘Sixty<br />
Something...’ study. Patient Educ Couns 19: 61–74<br />
Haas L, Maryniuk M, Beck J et al (2013) National Standards for<br />
Diabetes Self-Management Education and Support. Diabetes<br />
Care 36: S100–S108<br />
Norris SL, Lau J, Smith SJ et al (2002) Self-management education<br />
for adults with type 2 diabetes: a meta-analysis of the effect on<br />
glycemic control. Diabetes Care 25: 1159–71<br />
Peyrot M, Rubin RR, Funnell MM, Siminerio LM (2009) Access to<br />
diabetes self-management education: results of national surveys<br />
of patients educators and physicians. Diabetes Educ 35: 246–8,<br />
252–6, 258–63<br />
Huxley R, Barzi F, Woodward M (2006) Excess risk of fatal coronary<br />
heart disease associated with diabetes in men and women: metaanalysis<br />
of 37 prospective cohort studies. BMJ 332: 73–8<br />
IDF Diabetes Atlas Commitee (2015) IDF Diabetes Atlas. IDF<br />
Diabetes Atlas (7 th edition). International Diabetes Federation,<br />
Brussels, Belgium<br />
Seshasai SR, Kaptoge S, Thompson A et al (2011) Diabetes mellitus<br />
fasting glucose and risk of cause-specific death. New Engl J Med<br />
364: 829–41<br />
Skovlund SE, Peyrot M (2005) The Diabetes Attitudes Wishes<br />
and Needs (DAWN) Program: A new approach to improving<br />
outcomes of diabetes care. Diabetes Spectrum 18: 136–42<br />
Lowe JM, Mensch M, McElduff P et al (2009) Does an advanced<br />
insulin education programme improve outcomes and health<br />
service use for people with Type 2 diabetes? A 5-year follow-up<br />
of the Newcastle Empowerment course. Diabet Med 26: 1277–81<br />
UK Prospective Diabetes Study Group (1999) Quality of life in type 2<br />
diabetic patients is affected by complications but not by intensive<br />
policies to improve blood glucose or blood pressure control<br />
(UKPDS 37). Diabetes Care 22: 1125–36<br />
14 Diabetes & Primary Care Australia Vol 2 No 1 2017
Save the date:<br />
The 2 nd PCDSA<br />
National Conference<br />
29 th April 2017<br />
Melbourne, VIC<br />
The 2017 PCDSA conference will be held on 29 th April 2017 in<br />
Melbourne, VIC.<br />
The conference has been specifically designed for all primary care<br />
clinicians working in diabetes care, with the aims of:<br />
l Advancing education and learning in the field of diabetes<br />
healthcare.<br />
l Promoting best practice standards and clinically effective care in<br />
the management of diabetes.<br />
l Facilitating collaboration between health professionals to improve<br />
the quality of diabetes primary care across Australia.<br />
Program<br />
The 2017 PCDSA National Conference program will combine cutting-edge<br />
scientific content with practical clinical sessions, basing the education on<br />
much more that just knowing the guidelines. The distinguished panel of<br />
speakers will share their specialised experience in an environment conducive<br />
to optimal learning. Ample question time and the opportunity for audience<br />
participation will feature on the agenda.<br />
Steering committee<br />
• Clinical A/Prof Ralph Audehm<br />
• Dr Nicholas Forgione<br />
• Clinical A/Prof Mark Kennedy<br />
• Dr Gary Kilov<br />
• Dr Jo-Anne Manski-Nankervis<br />
• Dr Rajna Ogrin<br />
• Dr Suzane Ryan
CPD module<br />
Clinical vascular screening of the foot:<br />
For life and limb<br />
Sylvia McAra, Robert Trevethan, Lexin Wang, Paul Tinley<br />
Citation: McAra S, Trevethan R,<br />
Wang L, Tinley P (2017) Clinical<br />
vascular screening of the foot:<br />
For life and limb. Diabetes &<br />
Primary Care Australia 2: 16–24<br />
Article points<br />
1. This article presents evidence<br />
to inform clinical pedal vascular<br />
assessment with an update<br />
of concepts and practices.<br />
2. Peripheral arterial disease<br />
(PAD) is prevalent but<br />
underrecognised due to<br />
difficulties with effective<br />
clinical screening, particularly<br />
in at-risk groups.<br />
3. Enhanced awareness of PAD<br />
and the implementation<br />
of the most sensitive<br />
tests address barriers to<br />
clinical PAD screening.<br />
4. Peripheral vascular status<br />
is important as a marker<br />
of cardiovascular risk and<br />
predictor of mortality.<br />
5. Opportunities for preventing<br />
cardiovascular mortality<br />
exist by identifying<br />
asymptomatic PAD.<br />
Key words<br />
– Ankle and toe pressures<br />
– Cardiovascular risk<br />
– Doppler ultrasound<br />
– Peripheral arterial disease<br />
– Vascular assessment<br />
Authors<br />
See page 23 for author details.<br />
Peripheral arterial disease (PAD) is asymptomatic in 50–75% of cases and tends to be<br />
underdiagnosed due to the inherent difficulties in screening. Accurate peripheral vascular<br />
testing is particularly important for those at highest risk of PAD, including older people<br />
and people with diabetes, renal disease or a history of smoking. Unfortunately, commonly<br />
used tests for PAD have limited sensitivity in these most at-risk populations. This article<br />
provides guidance to support early detection of PAD using evidence-based clinical tests. It<br />
also contains a flowchart as a clinical guide and a set of recommendations concerning the<br />
measurement of toe pressures. More targeted screening can reduce morbidity and mortality<br />
rates in people with PAD who are at high risk of cardiovascular events and who often remain<br />
undiagnosed.<br />
Peripheral arterial disease (PAD) is a<br />
degenerative condition involving<br />
changes of the arterial walls and<br />
endothelial responses. Large epidemiological<br />
studies confirm the importance of PAD as an<br />
indicator for generalised atherosclerosis (Caro<br />
et al, 2005; Diehm et al, 2009). Low peripheral<br />
perfusion indicates the presence of widespread<br />
atheromatous disease (Greenland et al, 2001).<br />
Distal perfusion predicts the risk of pedal<br />
wounds and their healing potential (Sonter et<br />
al, 2014), but, of greater significance, strong<br />
links exist between PAD and cardiovascular<br />
disease (CVD; Hooi et al, 2004; Caro et al,<br />
2005). Advanced age, male gender, diabetes,<br />
renal disease and smoking are also associated<br />
with a higher prevalence and severity of PAD<br />
(Caro et al, 2005). The presence of PAD carries<br />
the same mortality risk as a previous myocardial<br />
infarction or stroke (Caro et al, 2005).<br />
Historically, symptoms and visual signs<br />
have been used as indicators to generate the<br />
index of suspicion for further clinical testing.<br />
However, this approach is flawed because PAD<br />
is asymptomatic in 50–75% of cases (Diehm<br />
et al, 2009) and most visual signs of vascular<br />
insufficiency are low in sensitivity for PAD<br />
screening (McGee and Boyko, 1998; Williams<br />
et al, 2005). The ankle–brachial index (ABI) has<br />
value in screening general populations, but loses<br />
sensitivity in proportion to an increasing degree<br />
of vessel stenosis, a primary pathophysiological<br />
manifestation of PAD (Xu et al, 2010). Absolute<br />
toe pressures and toe–brachial indices (TBIs)<br />
are now being recommended and are attracting<br />
research attention as adjuncts to improve the<br />
quality of PAD screening.<br />
Why screen for PAD?<br />
PAD is prevalent and often invisible, and it is<br />
underdiagnosed and commonly undertreated<br />
(Lange et al, 2004) due to barriers to screening<br />
(Haigh et al, 2013) and difficulties of<br />
recognition (Hirsch et al, 2001; Menz, 2010).<br />
A high proportion of cases of sudden cardiac<br />
death (25%) have no previously identified<br />
symptoms or appreciable risk factors of CVD<br />
(Greenland et al, 2001). There is, therefore, a<br />
need for tests and markers to assist clinicians in<br />
identifying people at risk (Aboyans and Criqui,<br />
16 Diabetes & Primary Care Australia Vol 2 No 1 2017
Clinical vascular screening of the foot<br />
2006; Aboyans et al, 2008; World Health<br />
Organization, 2013; Brownrigg et al, 2016),<br />
particularly when risks can be modified with<br />
interventions (Hinchcliffe et al, 2015).<br />
Prevalence, identification and<br />
classification of PAD<br />
Estimates of the prevalence of PAD vary<br />
widely from 4–57%, depending on how the<br />
disease is identified and on age and risk factor<br />
distributions in specific populations (Caro et<br />
al, 2005). In a summary statement about<br />
prevalence, Høyer et al (2013) cite evidence<br />
that more than 50% of people with PAD are<br />
asymptomatic.<br />
PAD is best known for ischaemic pain<br />
associated with intermittent claudication.<br />
However, in a large study, only 11% of people<br />
with PAD had intermittent claudication (Hirsch<br />
et al, 2001). The prevalence of pathology<br />
is similar in symptomatic and asymptomatic<br />
PAD (Diehm et al, 2009), but significant<br />
impairment of the vascular tree often exists<br />
before and without any symptoms or signs.<br />
Previously, the severity of PAD has been<br />
described and stratified using the symptoms<br />
of claudication and rest pain, then tissue<br />
death, as in the Rutherford and Fontaine<br />
classification systems (Mills et al, 2014). Due to<br />
a growing appreciation of both the prevalence<br />
and pathological significance of asymptomatic<br />
PAD, new international guidelines for vascular<br />
surgery contain recommendations that pedal<br />
risk stratification be based, instead of on<br />
symptoms, on an algorithm including foot<br />
wound status, ischaemia and infection (Mills<br />
et al, 2014).<br />
Standard CVD risk scores, such as the<br />
Framingham risk score, have low-, middleand<br />
high-risk stratification categories. The<br />
diagnostic utility of these indicators may be<br />
improved by adding non-invasive clinical pedal<br />
vascular assessment to identify asymptomatic<br />
PAD in the intermediate risk group (Greenland<br />
et al, 2001).<br />
Limitations in screening for PAD<br />
A major limitation associated with screening<br />
for PAD is that there is currently no agreement<br />
concerning the use of any single test or<br />
combination of tests to detect PAD in primary<br />
healthcare settings. People’s medical history, as<br />
well as their pulses, pedal Doppler waveforms,<br />
ABIs and TBIs, are quoted in guidelines as<br />
being strongly recommended, but there is little<br />
evidence to support their use (Hinchcliffe et<br />
al, 2015). Most clinical tests used for PAD<br />
screening have low sensitivity and therefore fail<br />
to identify a large proportion of people who have<br />
the disease (Williams et al, 2005; Brownrigg et<br />
al, 2016). Many people with PAD have no<br />
obvious visual signs, and visual signs such as<br />
skin colour, lack of hair growth, nail changes<br />
and skin atrophy are low in sensitivity for PAD<br />
detection (Williams et al, 2005; Menz, 2010).<br />
In addition to visual screening, standard clinical<br />
tests include assessment of pulses, impressions<br />
of skin temperature, capillary refilling time and<br />
possibly ABIs. These screening processes may<br />
underestimate PAD by up to 60% (Williams<br />
et al, 2005; Høyer et al, 2013). Pulse palpation,<br />
although a useful clinical skill, is not adequate<br />
as a primary screening tool for PAD due to its<br />
variable sensitivity, which declines as vascular<br />
disease states advance (McGee and Boyko,<br />
1998; Williams et al, 2005).<br />
The ABI has been the cornerstone of peripheral<br />
vascular assessment in primary care for PAD and<br />
associated CVD risk, and it is supported by four<br />
decades of evidence (Caruana et al, 2005; Rooke<br />
et al, 2011). However, when Australian GPs were<br />
surveyed for the barriers they experienced in<br />
performing vascular assessment, 58% indicated<br />
that they did not use ABIs to perform vascular<br />
assessments, with time constraints stated as the<br />
greatest barrier, followed by lack of equipment<br />
and skills (Haigh et al, 2013). This is despite<br />
Medicare rebates currently applying for both<br />
toe- and ankle-pressure studies (See Table 1).<br />
The ABI is useful for identifying CVD<br />
risk in the general population (Caruana et al,<br />
2005; Guo et al, 2008). However, its sensitivity<br />
is reduced in proportion to the degree of<br />
atherosclerosis and vascular stenosis, both of<br />
which are common in people of advanced age<br />
and those who have complications of diabetes,<br />
especially neuropathy (Aboyans et al, 2008; Xu<br />
et al, 2010; Craike et al, 2013; Formosa et al,<br />
Page points<br />
1. Estimates of the prevalence<br />
of peripheral arterial disease<br />
(PAD) vary widely from 4–57%,<br />
depending on how the disease<br />
is identified and on age and risk<br />
factor distributions in specific<br />
populations.<br />
2. A major limitation associated<br />
with screening for PAD is that<br />
there is currently no agreement<br />
concerning the use of any<br />
single test or combination of<br />
tests to detect PAD in primary<br />
healthcare settings.<br />
Diabetes & Primary Care Australia Vol 2 No 1 2017 17
Clinical vascular screening of the foot<br />
Table 1. Medicare fees and benefits for vascular testing (Australian Government Department of Health, 2016).<br />
Test<br />
Ankle– or toe–brachial index and arterial waveform study<br />
Measurement of ankle:brachial indices and arterial waveform analysis<br />
Measurement of posterior tibial and dorsalis pedis (or toe) and brachial arterial pressures bilaterally using Doppler or<br />
plethysmographic techniques, the calculation of ankle (or toe)–brachial systolic pressure indices and assessment of arterial<br />
waveforms for the evaluation of lower extremity arterial disease, examination, hard copy trace and report.<br />
Ankle– or toe–brachial index-exercise study<br />
Exercise study for the evaluation of lower extremity arterial disease<br />
Measurement of posterior tibial and dorsalis pedis (or toe) and brachial arterial pressures bilaterally using Doppler or<br />
plethysmographic techniques, the calculation of ankle (or toe) brachial systolic pressure indices for the evaluation of lower<br />
extremity arterial disease at rest and following exercise using a treadmill or bicycle ergometer or other such equipment<br />
where the exercise workload is quantifiably documented, examination and report.<br />
Item<br />
number<br />
11610<br />
11612<br />
Fees and Medicare<br />
benefits<br />
Fee: $63.75<br />
Benefit: 75% = $47.85<br />
85% = $54.20<br />
Fee: $112.40<br />
Benefit: 75% = $84.30<br />
85% = $95.55<br />
2013; Hyun et al, 2014).<br />
Medial arterial calcification is prevalent in<br />
renal disease (An et al, 2010) and in longterm<br />
type 1 diabetes (Ix et al, 2012). It places<br />
limitations on the sensitivity of vascular<br />
pressure measurements due to the associated<br />
non-compressibility of vessels.<br />
Sensitive clinical screening methods<br />
Buerger’s sign demonstrates the pathophysiology<br />
of endothelial-driven maximal vasodilation of<br />
vessels in the presence of tissue ischaemia,<br />
resulting in pallor on elevation from rapid and<br />
extensive draining, and rubor on dependency<br />
with gravity-assisted refill of dilated vessels<br />
(Figure 1). Buerger’s sign has high sensitivity,<br />
up to 100% in severe arterial disease (McGee<br />
and Boyko, 1998). It, therefore, holds an<br />
important place in the PAD screening armoury.<br />
A degree of sensitive clinical screening can<br />
also be achieved by assessing pedal arteries<br />
by means of handheld Doppler ultrasound<br />
a. b.<br />
Figure 1. Buerger’s sign: pallor in elevation (a), rubor in dependency (b). Colour change is notable within 10 seconds of position change. Buerger’s sign<br />
is the only visual sign that is highly sensitive for peripheral arterial disease screening. See also http://bit.ly/2gUqGzS (Kang and Chung, 2007 [accessed<br />
05.12.16]).<br />
18 Diabetes & Primary Care Australia Vol 2 No 1 2017
Clinical vascular screening of the foot<br />
and waveform analysis ([Figure 2] as distinct<br />
from laboratory Doppler ultrasound colour<br />
imaging). Sound and waveform analysis is a<br />
good indicator of pathology and has prognostic<br />
relevance, and it also maintains sensitivity<br />
in the presence of neuropathy (Williams<br />
et al, 2005; Alavi et al, 2015). Although<br />
some ambiguity is associated with biphasic<br />
and triphasic Doppler signals (both can be<br />
confounded by a variety of influences including<br />
flow turbulence and valvular incompetence),<br />
monophasic Doppler signals are highly sensitive<br />
indicators of significant vascular pathology.<br />
Doppler analysis can be performed in the same<br />
brief time period needed for other auscultation<br />
techniques. Investigations of the sensitivity<br />
of the ABI versus Doppler ultrasound and<br />
waveforms in PAD screening endorse Doppler<br />
and document the limitations of the reliability<br />
of the ABI alone (Formosa et al, 2013; Alavi<br />
et al, 2015).<br />
Additional prospects for effective screening<br />
are offered by TBIs. In a recent systematic<br />
review based on seven studies, Tehan et al<br />
(2016) found that the sensitivity of TBIs for<br />
detecting PAD ranged from 45% to 100%,<br />
with TBIs being most sensitive in samples<br />
known to be at risk of PAD and among people<br />
who experienced intermittent claudication.<br />
The small number of studies reviewed were<br />
of varying quality and comprised disparate<br />
samples, indicating the need for more extensive,<br />
systematic and rigorous investigation regarding<br />
the effectiveness of TBIs for detecting PAD.<br />
Nevertheless, use of the TBI in place of the<br />
ABI is recommended because of the TBI’s<br />
superior sensitivity among people who have<br />
known vascular disease risk – specifically<br />
people with diabetes and renal disease and of<br />
advanced age (Williams et al, 2005; Aboyans<br />
et al, 2008; Hyun et al, 2014). An alternative<br />
assessment algorithm incorporating Doppler<br />
ultrasound waveform analysis and TBIs for<br />
people with diabetes has been found to increase<br />
the sensitivity for PAD detection from 33% to<br />
50% (Craike and Chuter, 2015).<br />
When vascular disease is widespread and<br />
other comorbidities (such as cardiac output<br />
disorders, respiratory disease and diabetes)<br />
Figure 2. The presence of a monophasic signal in a pedal artery from handheld Doppler<br />
ultrasound is a highly sensitive indicator of peripheral arterial disease. Tibialis posterior, dorsalis<br />
pedis and tibialis anterior arteries are useful for auscultation and the test can be performed in<br />
less than a minute.<br />
complicate systemic pressure measurements,<br />
absolute toe pressures are probably more<br />
valuable than are indices such as ABIs and<br />
TBIs (Caruana et al, 2005; Potier et al, 2011;<br />
Okada et al, 2015; McAra and Trevethan,<br />
2016).<br />
The use of X-ray should be considered as a<br />
novel and important part of PAD screening<br />
because both toe and ankle vessels may<br />
become calcified, and, as a result, pressure<br />
measurement can be spuriously inflated due to<br />
vessel non-compressibility. By identifying the<br />
presence of calcification, X-rays provide a more<br />
informed context within which to interpret<br />
toe and ankle pressures. In a study of people<br />
with type 1 diabetes, the incidence of medial<br />
arterial calcification was 57% on plain X-ray,<br />
but only 8% of these people had ABIs >1.30 (Ix<br />
et al, 2012), demonstrating not only the value<br />
of X-ray, but also, as the researchers concluded,<br />
that the ABI should not to be relied on for<br />
identifying PAD due to its underdiagnosis of<br />
the disease.<br />
More research is needed about the prevalence<br />
of toe calcification to sharpen understanding of<br />
the utility of toe pressures in groups at highest<br />
risk of PAD. However, it is already known that<br />
Diabetes & Primary Care Australia Vol 2 No 1 2017 19
Clinical vascular screening of the foot<br />
toes are affected by calcification later than are<br />
ankles and only in cases of the most severe and<br />
long-standing disease.<br />
There is consensus that larger-scale studies are<br />
needed to consolidate normal and pathological<br />
TBI ranges and recommendations for TBI and<br />
toe pressure test procedures (Høyer et al, 2013;<br />
Sonter et al, 2014; McAra and Trevethan, 2016;<br />
Tehan et al, 2016). However, there is evidence<br />
that the most sensitive screening procedures<br />
parallel the most accurate prognostic indicators.<br />
Toe pressures and TBIs have been linked to<br />
amputation prognosis by a systematic review<br />
of the literature, which indicated that there is a<br />
3.25 times greater risk of non-healing when toe<br />
pressures are 10 mmHg<br />
Abnormal result<br />
Repeat measurement +<br />
medical referral +<br />
reassessments including<br />
X-ray and vascular<br />
imaging<br />
Borderline result<br />
Repeat measurements +<br />
document +<br />
routine reassessments<br />
Normal result<br />
Routine reassessment:<br />
Annual vascular review<br />
if over 65 or over 50<br />
with other risk factors<br />
Figure 3. Pedal vascular assessment guide. ABI=ankle–brachial index; PAD=Peripheral arterial disease;<br />
TBI=toe–brachial index.<br />
20 Diabetes & Primary Care Australia Vol 2 No 1 2017
Clinical vascular screening of the foot<br />
Measurement of vascular pressures<br />
Brachial pressures: Why check for inter-arm<br />
differences?<br />
Brachial blood pressures should ideally be<br />
taken in both arms, particularly in people at<br />
risk of PAD, as inter-arm differences in brachial<br />
blood pressure can predict mortality. In a<br />
recent meta-analysis (Clark et al, 2012), interarm<br />
differences >10 mmHg were shown to be a<br />
marker of cardiovascular mortality. Beyond an<br />
initial 10 mmHg inter-arm difference, every<br />
additional 1 mmHg difference accounted for<br />
a 5% greater hazard ratio when the CVD risk<br />
score was >20%. Obtaining brachial blood<br />
pressures provides an opportunity for health<br />
professionals involved in vascular screening<br />
to identify and assess people for appropriate<br />
medical management, including investigation<br />
and targeting of CVD risk factors.<br />
Lower limb pressure measurement<br />
considerations<br />
Lower limb vascular pressure measurements are<br />
known to be variable and subject to influence<br />
by numerous factors, including ambient and<br />
skin temperatures, length of any rest period<br />
before measurement, respiratory and cardiac<br />
outputs, the comfort of the person being<br />
tested, medications and cuff sizes (Påhlsson<br />
et al, 2004; Welch Allyn Inc, 2010; Sadler<br />
et al, 2014; Sonter et al, 2014; McAra and<br />
Trevethan, 2016). Some recommendations<br />
regarding measurement of blood pressure in<br />
toes are summarised in Box 1.<br />
One of the most important test conditions,<br />
and most pertinent to lower limb testing,<br />
is the relative position of the test segment.<br />
Lying with heart and foot at the same level<br />
is the ideal measurement position (Figure 4).<br />
Any elevation of the limb relative to the heart<br />
markedly decreases pressures (Welch Allyn<br />
Inc, 2010) and the reduction is in proportion<br />
to vascular impairment (Wiger and Styf,<br />
1998). This physiological principle, which is<br />
magnified in PAD and evident in Buerger’s<br />
sign, has an immediate influence on pressure<br />
“Lower limb vascular<br />
pressure measurements<br />
are known to be<br />
variable and subject<br />
to influence by<br />
numerous factors.”<br />
Box 1. Recommendations for toe pressure measurement.<br />
l Assess toe pressures in an ambient temperature between 21 and 24°C (Bonham, 2011).<br />
l Be aware that elevated blood pressure readings are likely if people being tested have a full bladder or have eaten, consumed<br />
caffeinated or alcoholic beverages, smoked, or engaged in vigorous physical activity within an hour of testing (Pickering et al,<br />
2005).<br />
l Place the person in a supine position with the heart, arms and feet at the same level (Bonham, 2011). Consider elevating the head<br />
only with one or two pillows for comfort. If taking a brachial pressure, place the arm on a pillow to bring it up to the same level as<br />
the heart (Pickering et al, 2005).<br />
l Provide an initial 10 minutes’ rest period of sitting or lying (Sadler et al, 2014), preferably lying.<br />
l Ensure skin temperature is at least 19°C (Cloete et al, 2009). Use some form of warming if necessary.<br />
l Avoid perturbations such as the subject’s talking, moving, coughing or sneezing (McAra and Trevethan, 2016).<br />
l Use photoplethysmography (PPG) as the sensing method, preferably using an automated device (Pérez Martin et al, 2010).<br />
l Use an occlusion cuff of 2.5 cm if possible; if smaller cuffs are used, allow for the possibility of inflated readings (Påhlsson et al,<br />
2004).<br />
l If measuring toe–brachial indices (TBIs), for each limb, take two readings of brachial systolic pressures and toe systolic pressures<br />
and, for each limb, average the readings if they are similar to each other. However, if they differ noticeably, take three or<br />
more readings and make a judicious decision about which ones should be averaged. Obtain brachial and pedal readings as<br />
simultaneously as possible (McAra and Trevethan, 2016).<br />
l Raise a high index of suspicion of peripheral arterial disease with a TBI reading of
Clinical vascular screening of the foot<br />
a. b.<br />
Figure 4. Positioning for vascular pressure measurement requires the heart and the sites to be measured on the same horizontal plane. Flat lying is ideal (a).<br />
Note the pillows for the head and the brachium (Pickering et al, 2005). The angled chair allows for correct alignment when flat lying is not practical due to<br />
the person’s conditions (b).<br />
“Attention to test<br />
conditions and<br />
awareness of<br />
pathophysiology<br />
associated with<br />
peripheral arterial<br />
disease can lead<br />
to more effective<br />
screening.”<br />
readings as formalised with the pole test<br />
(Menz, 2010). As well as segment positioning<br />
being an important principle affecting accurate<br />
measurement, it extends to management:<br />
positioning of the foot in relative dependency<br />
may boost supply and thereby assist in arterial<br />
wound healing and the relief of rest pain.<br />
The issue of cuff size for toe pressures is<br />
important and has been underappreciated in<br />
the literature to date. Smaller cuff sizes have<br />
been demonstrated to produce higher blood<br />
pressure values (Påhlsson et al, 2004), and<br />
this can present problems, particularly as<br />
automated twin-cuff devices frequently require<br />
the use of a smaller occlusion cuff to fit the<br />
toe (McAra and Trevethan, 2016). As a result<br />
of commonly found fluctuations in vascular<br />
pressures, particularly brachial pressures in<br />
diabetes, repeat and serial testing of pedal<br />
pressures and indices is recommended (Sonter<br />
et al, 2014; McAra and Trevethan 2016).<br />
Conclusions<br />
l Effective PAD identification in primary<br />
clinical contact settings can improve<br />
disease identification and monitoring, and,<br />
importantly, CVD-risk modification.<br />
l Reliance on tests with low sensitivity has<br />
pervaded understanding and practice in the<br />
identification of PAD. This has contributed<br />
to a substantial proportion of missed<br />
diagnoses.<br />
l The ABI has fulfilled a valuable role in<br />
screening for PAD and CVD in the general<br />
population. However, ABI sensitivity<br />
declines substantially in populations at the<br />
highest risk of PAD and CVD when vessel<br />
stenosis becomes prevalent.<br />
l The most sensitive clinical options for<br />
PAD screening in at-risk populations<br />
are Buerger’s sign, Doppler ultrasound<br />
waveforms and, more recently, toe<br />
pressures (including TBIs). X-rays can<br />
assist in identifying vessel calcification,<br />
thus providing important information for<br />
interpreting vascular pressure values.<br />
l Time saved by avoiding less sensitive clinical<br />
assessments could be used to conduct more<br />
sensitive screening procedures.<br />
l Attention to test conditions and awareness<br />
of pathophysiology associated with PAD can<br />
lead to more effective screening. n<br />
Competing interests<br />
No competing interests to declare.<br />
Acknowledgements<br />
Richard Barkas, Martin Forbes, Rajna Ogrin, Barry<br />
Pitman and Caroline Robinson provided helpful<br />
suggestions, comments and advice concerning<br />
earlier drafts of this manuscript.<br />
22 Diabetes & Primary Care Australia Vol 2 No 1 2017
Clinical vascular screening of the foot<br />
Aboyans V, Criqui M (2006) Can we improve cardiovascular risk<br />
prediction beyond risk equations in the physician’s office? J Clin<br />
Epidemiol 59: 547–58<br />
Aboyans V, Ho E, Denenberg JO, Ho LA et al (2008) The<br />
association between elevated ankle systolic pressures and<br />
peripheral occlusive arterial disease in diabetic and nondiabetic<br />
subjects. J Vasc Surg 48: 1197–203<br />
Alavi A, Sibbald RG, Nabavizadeh R et al (2015) Audible handheld<br />
Doppler ultrasound determines reliable and inexpensive<br />
exclusion of significant peripheral arterial disease. Vascular 23:<br />
622–9<br />
An WS, Son YK, Kim S-E et al (2010) Vascular calcification score<br />
on plain radiographs of the feet as a predictor of peripheral<br />
arterial disease in patients with chronic kidney disease. Int Urol<br />
Nephrol 42: 773–80<br />
Australian Government Department of Health (2016) Medicare<br />
Benefits Schedule Book. Commonwealth of Australia, Canberra,<br />
ACT<br />
Bonham PA (2011) Measuring toe pressures using a portable<br />
photoplethysmograph to detect arterial disease in high-risk<br />
patients: an overview of the literature. Ostomy Wound Manag<br />
57: 36–44<br />
Brownrigg RJ, Hinchliffe R, Apelqvist J et al (2016) Effectiveness<br />
of bedside investigations to diagnose peripheral artery disease<br />
among people with diabetes mellitus: a systematic review.<br />
Diabetes Metab Res Rev 32(Suppl 1): 119–27<br />
Caro J, Migliaccio-Walle K, Ishak K, Proskorovsky I (2005) The<br />
morbidity and mortality following a diagnosis of peripheral<br />
arterial disease: long-term follow-up of a large database. BMC<br />
Cardiovasc Disord 5: 14<br />
Caruana MF, Bradbury AW, Adam DJ (2005) The validity, reliability,<br />
reproducibility and extended utility of ankle to brachial pressure<br />
index in current vascular surgical practice. Eur J Vasc Endovasc<br />
Surg 29: 443–51<br />
Clark C, Taylor R, Shore A et al (2012) Association of a difference<br />
in systolic blood pressure between arms with vascular disease<br />
and mortality: a systematic review and meta-analysis. Lancet<br />
379: 905–14<br />
Cloete N, Kiely C, Colgan M et al (2009) Reproducibility of toe<br />
pressure measurements. J Vasc Ultrasound 33: 129–32<br />
Craike P, Chuter V, Bray A et al (2013) The sensitivity and<br />
specificity of the toe-brachial index in detecting peripheral<br />
arterial disease. J Foot Ankle Res 6(Suppl 1): 3<br />
Craike P, Chuter V (2015) A targeted screening method for<br />
peripheral arterial disease: a pilot study. J Foot Ankle Res<br />
8(Suppl 2): O8<br />
Diehm C, Allenberg J, Pittrow D et al (2009) Mortality and<br />
vascular morbidity in older adults with asymptomatic versus<br />
symptomatic peripheral artery disease. Circulation 1: 2053–61<br />
Formosa C, Cassar K, Gatt A et al (2013) Hidden dangers revealed<br />
by misdiagnosed peripheral arterial disease using ABPI<br />
measurement. Diabetes Res Clin Pract 102: 112–6<br />
Greenland P, Smith SC, Grundy S (2001) Improving coronary<br />
heart disease risk assessment in asymptomatic people – Role<br />
of traditional risk factors and noninvasive cardiovascular tests.<br />
Circulation 104: 1863–7<br />
Guo X, Li J, Pang W, Zhao M et al (2008) Sensitivity and specificity<br />
of ankle-brachial index for detecting angiographic stenosis of<br />
peripheral arteries. Circ J 72: 605–10<br />
Haigh K, Bingley J, Golledge J, Walker PJ (2013) Barriers to<br />
screening and diagnosis of peripheral artery disease b y general<br />
practitioners. Vasc Med 18: 325–30<br />
Hinchcliffe RJ, Brownrigg JRW, Apelqvist J et al (2015) IWGDF<br />
Guidance on the diagnosis, prognosis and management of<br />
peripheral artery disease in patients with foot ulcers in diabetes.<br />
IWGDF Working Group on Peripheral Artery Disease, London,<br />
UK. Available at: http://iwgdf.org/guidelines/guidance-onpad-2015<br />
(accessed 17.11.16)<br />
Hirsch AT, Criqui MH, Treat-Jacobson D et al (2001) Peripheral<br />
arterial disease detection, awareness, and treatment in primary<br />
care. JAMA 286: 1317–24<br />
Hooi JD, Kester ADM, Stoffers HEJH et al (2004) Asymptomatic<br />
peripheral arterial occlusive disease predicted cardiovascular<br />
morbidity and mortality in a 7-year follow-up study. J Clin<br />
Epidemiol 57: 294–300<br />
Høyer C, Sandermann J, Petersen LJ (2013) The toe-brachial index<br />
in the diagnosis of peripheral arterial disease. J Vasc Surg 58:<br />
231–8<br />
Hyun S, Forbang NI, Allison MA et al (2014) Ankle-brachial index,<br />
toe-brachial index, and cardiovascular mortality in persons with<br />
and without diabetes mellitus. J Vasc Surg 60: 390–5<br />
Ix J, Miller R, Criqui M, Orchard TJ (2012) Test characteristics of the<br />
ankle-brachial index and ankle-brachial difference for medial<br />
arterial calcification on X-ray in type 1 diabetes. J Vasc Surg 56:<br />
721–7<br />
Kang H, Chung M (2007) Images in peripheral artery disease.<br />
N Engl J Med 357 (e19)<br />
Lange S, Diehm C, Darius H et al (2004) High prevalence of<br />
peripheral arterial disease and low treatment rates in elderly<br />
primary care patients with diabetes. Exp Clin Endocrinol<br />
Diabetes 112: 566–73<br />
McAra S, Trevethan R (2016) Measurement of toe-brachial indices<br />
in people with subnormal toe pressures: complexities and<br />
revelations. J Am Podiatr Med Assoc [In press]<br />
McGee SR, Boyko EJ (1998) Physical examination and chronic<br />
lower-extremity ischemia. A critical review. Arch Intern Med<br />
158: 1357–64<br />
Menz H (2010) Foot problems in older people. Elsevier,<br />
Philadelphia, USA<br />
Mills LR, Conte MS, Armstrong DG et al (2014) The Society<br />
for Vascular Surgery Lower Extremity Threatened Limb<br />
Classification System: Risk stratification based on wound,<br />
ischemia, and foot infection (WIfI). J Vasc Surg 59: 220–3<br />
Okada R, Yasuda Y, Tsushita K et al (2015) Within-visit blood<br />
pressure variability is associated with prediabetes and diabetes.<br />
Scientific Reports 5: 7964<br />
Påhlsson HI, Jorneskog G, Wahlberg E (2004) The cuff width<br />
influences the toe blood pressure value. Vasa 33: 215–8<br />
Pérez-Martin A, Meyer G, Demattei C et al (2010) Validation of a<br />
fully automatic photoplethysmographic device for toe blood<br />
pressure measurement. Eur J Vasc Endovasc Surg 40: 515–20<br />
Pickering TG, Hall JE, Appel LJ et al (2005) Recommendations<br />
for blood pressure measurement in humans and experimental<br />
animals. Part 1: Blood pressure measurement in humans:<br />
a statement for professionals from the subcommittee of<br />
professional and public education of the American Heart<br />
Association Council on high blood pressure research.<br />
Circulation 111: 697–716<br />
Potier L, Abi Khalil C, Mohammedi K, Roussel R (2011) Use and<br />
utility of ankle-brachial index in patients with diabetes. Eur J<br />
Vasc Endovasc Surg 41: 110–6<br />
Rooke T, Hirsch A, Misra S et al (2011) ACCF/AHA Focused update<br />
of the guideline for the management of patients with peripheral<br />
artery disease (Updating the (2005 Guideline): a report of the<br />
American College of Cardiology Foundation/American Heart<br />
Association task force on practice guidelines. The Society<br />
for Cardiovascular Angiography and Interventions. J Am Coll<br />
Cardiol 58: 2020–45<br />
Sadler S, Chuter V, Hawke F (2014) A systematic review of the<br />
effect of pre-test rest duration on toe and ankle systolic blood<br />
pressure measurements. BMC Res Notes 7(213)<br />
Sonter JA, Ho A, Chuter VH (2014) The predictive capacity of<br />
toe blood pressure and the toe-brachial index for foot wound<br />
healing and amputation: a systematic review and meta analysis.<br />
Wound Practice and Research 22: 208–20<br />
Tehan P, Santos D, Chuter V (2016) A systematic review of the<br />
sensitivity and specificity of the toe-brachial index for detecting<br />
peripheral artery disease. Vasc Med 21: 382–90<br />
Welch Allyn Inc (2010) Blood pressure accuracy and variability<br />
quick reference. Welch Allyn Inc, NY, USA. Available at:<br />
http://bit.ly/2g1SNvP (accessed 18.11.16)<br />
Wiger P, Styf JR (1998) Effects of limb elevation on abnormally<br />
increased intramuscular pressure, blood perfusion pressure,<br />
and foot sensation: an experimental study in humans. J Orthop<br />
Trauma 12: 343–7<br />
Williams D, Harding K, Price P (2005) An evaluation of the efficacy<br />
of methods used in screening for lower-limb arterial disease in<br />
diabetes. Diabetes Care 28: 2206–10<br />
World Health Organization (2013) The top 10 causes of death.<br />
Fact sheet no. 310. WHO, Geneva, Switzerland. Available at:<br />
http://bit.ly/1c9a3vO (accessed 17.11.16)<br />
Xu D, Li J, Zou L et al (2010) Sensitivity and specificity of the<br />
ankle-brachial index to diagnose peripheral artery disease: a<br />
structured review. Vasc Med 15: 361–9<br />
Authors<br />
Sylvia McAra, Adjunct Lecturer,<br />
Podiatry, School of Community<br />
Health, Charles Sturt University,<br />
Thurgoona, NSW; Robert<br />
Trevethan, Independent academic<br />
researcher and author, Albury,<br />
NSW; Lexin Wang, Professor of<br />
Clincial Pharmacology, School<br />
of Biomedical Sciences, Charles<br />
Sturt University, Wagga Wagga,<br />
NSW; Paul Tinley Associate<br />
Professor, Course Coordinator,<br />
Podiatry, School of Community<br />
Health, Charles Sturt University,<br />
Thurgoona, NSW.<br />
Diabetes & Primary Care Australia Vol 2 No 1 2017 23
Clinical vascular screening of the foot<br />
Online CPD activity<br />
Visit www.pcdsa.com.au/cpd to record your answers and gain a certificate of participation<br />
Participants should read the preceding article before answering the multiple choice questions below. There is ONE correct answer to each question.<br />
After submitting your answers online, you will be immediately notified of your score. A pass mark of 70% is required to obtain a certificate of<br />
successful participation; however, it is possible to take the test a maximum of three times. A short explanation of the correct answer is provided.<br />
Before accessing your certificate, you will be given the opportunity to evaluate the activity and reflect on the module, stating how you will use what<br />
you have learnt in practice. The CPD centre keeps a record of your CPD activities and provides the option to add items to an action plan, which will<br />
help you to collate evidence for your annual appraisal.<br />
1. In what percentage of sudden cardiac<br />
deaths are there no previously determined<br />
cardiovascular risk factors? Select ONE<br />
option only.<br />
A. 1%<br />
B. 10%<br />
C. 25%<br />
D. 50%<br />
2. The risk of mortality with peripheral<br />
arterial disease (PAD) is similar to the risk<br />
of mortality in which of the following<br />
groups of people? Select ONE option only.<br />
A. People who smoke cigarettes<br />
B. People who have diabetes<br />
C. People with a history of a previous<br />
cardiovascular or cerebrovascular event<br />
D. People with renal disease<br />
3. According to Caro et al (2005), what is<br />
the estimated range of prevalence of PAD?<br />
Select ONE option only.<br />
5. Of the visual signs of PAD, which of the<br />
following has the highest sensitivity?<br />
Select ONE option only.<br />
A. Colour<br />
B. Pallor<br />
C. Skin atrophy<br />
D. Buerger’s sign<br />
6. The ankle–brachial index (ABI) is useful<br />
for identifying cardiovascular disease risk<br />
in the general population; however, it has<br />
reduced sensitivity in proportion to the<br />
degree of vascular stenosis present in an<br />
individual. Which of the following factors<br />
reduces the sensitivity of the ABI for PAD<br />
screening? Select ONE option only.<br />
A. Diabetes<br />
B. Neuropathy<br />
C. Renal disease<br />
D. Advanced age<br />
E. All of the above<br />
8. In a recent meta-analysis of inter-arm<br />
differences in brachial systolic blood<br />
pressures (Clarke et al, 2012), what was<br />
the threshold shown to be a marker of<br />
cardiovascular mortality in the presence<br />
of a CVD risk score of >20%? Select ONE<br />
option only.<br />
A. A 45 mmHg difference<br />
B. A 25 mmHg difference<br />
C. A 10 mmHg difference<br />
D. A 5 mmHg difference<br />
9. Using a handheld Doppler ultrasound, the<br />
result of a monophasic signal (sound or<br />
waveform) is indicative of the following<br />
diagnosis? Select ONE option only.<br />
A. A normal result<br />
B. PAD is highly likely<br />
C. An ambiguous outcome with regard<br />
to PAD<br />
D. PAD is very unlikely<br />
A.
Article<br />
Antimicrobial management of<br />
diabetic foot infection<br />
Roy Rasalam, Caroline McIntosh, Aonghus O’Loughlin<br />
Diabetic foot infections (DFIs) are a frequent and costly complication associated<br />
with diabetes mellitus. Osteomyelitis is present in 44–68% of patients admitted to<br />
hospital and DFIs account for 60% of lower extremity amputations in developed<br />
countries. Diagnosis of DFIs should be based upon the presence of local and<br />
systemic signs and symptoms, and the management and outcomes of DFIs are<br />
superior through the involvement of a multidisciplinary team. This article presents<br />
an overview of the current evidence for diagnosis and management of DFIs in<br />
practice.<br />
The prevalence of diabetes mellitus has<br />
increased dramatically in recent decades,<br />
as have the complications associated<br />
with the condition (Lipsky et al, 2016). It<br />
is estimated there are currently 415 million,<br />
or one in 11, people with diabetes globally<br />
(International Diabetes Federation, 2015).<br />
Chronic hyperglycaemia associated with diabetes<br />
mellitus is known to have a detrimental effect on<br />
human immune function; specifically, cellular<br />
immunity and polymorphonuclear leukocytes<br />
are affected, and phagocytosis is impaired (Akkus<br />
et al, 2016). Thus, people with diabetes are at<br />
increased risk of diabetic foot infections (DFIs).<br />
According to Peters (2016), the incidence of foot<br />
infections in people with diabetes ranges from an<br />
overall lifetime risk of 4% to a yearly risk of 7%.<br />
Additionally, approximately 60% of diabetic<br />
foot ulcers (DFUs) are infected on presentation<br />
(Markakis et al, 2016). DFIs usually occur when<br />
pathogens enter the foot through a break in<br />
the skin’s integrity, such as a neuropathic or<br />
neuroischaemic foot ulceration (Peters, 2016).<br />
DFIs are associated with significant morbidity<br />
and mortality, as infection can spread rapidly in<br />
the diabetic foot. If infection spreads to deeper<br />
structures, including the underlying bone, diabetic<br />
foot osteomyelitis (DFO) develops. DFIs are<br />
the most frequent diabetes-related complication<br />
requiring hospitalisation, and DFO is present in<br />
44–68% of cases admitted to hospital (Lipsky<br />
et al, 2016; Peters, 2016). If not managed<br />
appropriately, DFI can trigger a cascade that<br />
results in lower extremity amputation, especially in<br />
people with peripheral arterial disease (Markakis<br />
et al, 2016). DFIs account for 60% of lower<br />
extremity amputations in developed countries<br />
(Peters, 2016). Prompt identification, rapid<br />
diagnosis, timely referral for specialist review<br />
and appropriate management strategies are all<br />
vital steps in the quest to minimise the adverse<br />
outcomes associated with DFIs.<br />
Establishing the diagnosis<br />
Peters (2016) stresses the importance of an<br />
initial diagnosis of DFI based on clinical signs<br />
and symptoms, as the reliance on bloods,<br />
microbiological and radiological studies could<br />
lead to a delay in diagnosis. However, he also<br />
acknowledges the challenges faced by clinicians<br />
when using clinical judgement. It is possible<br />
that the signs and symptoms of infection are less<br />
prevalent in people with diabetes. This may be<br />
due to the presence of foot ischaemia, neuropathy<br />
and immunopathy, which could, theoretically,<br />
reduce the inflammatory response and mask the<br />
classic signs of infection (Peters, 2016). Though as<br />
Peters (2016) highlights, this theory is not proven.<br />
Citation: Rasalam R, McIntosh C,<br />
O’Loughlin A (2017) Antimicrobial<br />
management of diabetic foot<br />
infection. Diabetes & Primary<br />
Care Australia 2: 25–9<br />
Article points<br />
1. Diabetic foot infections<br />
(DFIs) are the most frequent<br />
diabetes-related complications<br />
requiring hospitalisation.<br />
2. Approximately 60% of<br />
diabetic foot ulcers are<br />
infected on presentation.<br />
3. Osteomyelitis is present in<br />
44–68% of patients admitted<br />
into hospital with DFIs.<br />
4. DFIs account for 60% of<br />
lower extremity amputations<br />
in developed countries.<br />
5. Diagnosis of DFIs should be<br />
based upon the presence<br />
of local and systemic<br />
signs and symptoms.<br />
6. The management and<br />
outcome for a DFI is superior<br />
if there is the involvement of<br />
a multidisciplinary team.<br />
Key words<br />
– Antimicrobials<br />
– Diabetic foot infections<br />
– Diagnosis<br />
– Management<br />
Authors<br />
Roy Rasalam is Head of Clinical<br />
Skills & Medical Director,<br />
College of Medicine, James<br />
Cook University, Townsville<br />
City, Qld, Australia; Caroline<br />
McIntosh is Professor of Podiatric<br />
Medicine, Discipline of Podiatric<br />
Medicine, National University<br />
of Ireland Galway, Galway,<br />
Ireland; Aonghus O’Loughlin is<br />
Consultant Physician, Diabetes<br />
Day Centre, Galway University<br />
Hospital, Galway, Ireland.<br />
Diabetes & Primary Care Australia Vol 2 No 1 2017 25
Antimicrobial management of diabetic foot infection<br />
Table 1. Infectious Diseases Society of America (IDSA) and International<br />
Working Group on the Diabetic Foot classification of diabetic foot infection<br />
(adapted from Lipsky et al, 2012).<br />
Clinical manifestation<br />
of infection<br />
No symptoms or signs of<br />
infection<br />
Infection present (defined<br />
by the presence of two or<br />
more signs/symptoms):<br />
l Local swelling or<br />
induration<br />
l Erythema<br />
l Local tenderness or pain<br />
l Local warmth<br />
l Purulent discharge<br />
IDSA infection severity<br />
Not infected<br />
Mild<br />
Local infection involving only the skin and subcutaneous tissues.<br />
Moderate<br />
Local infection with erythema >2 cm or involving structures deeper<br />
than skin and subcutaneous tissues (e.g. abscess, osteomyelitis, septic<br />
arthritis, fasciitis), AND no systemic inflammatory response signs (as<br />
described below).<br />
Severe<br />
Local infection (as described above) with the signs of SIRS, as<br />
manifested by ≥2 of the following:<br />
l Temperature >38 o C or 90 bpm<br />
SIRS=Systemic inflammatory response syndrome.<br />
l Respiratory rate >20 breaths/min or PaCO 2<br />
12 000 or
Antimicrobial management of diabetic foot infection<br />
Table 2. Suggested route, setting and duration of antibiotic therapy by severity of extent of infection (adapted from Lipsky et al,<br />
2016).<br />
Severity or extent of infection Route of administration Setting Duration of therapy<br />
Soft-tissue only<br />
Mild Topical or oral Outpatient<br />
1–2 weeks (may extend up to 4 weeks<br />
if slow to resolve)<br />
Moderate Oral (or initial parenteral) Outpatient/inpatient 1–3 weeks<br />
Severe<br />
Initial parenteral, switch to oral<br />
when possible<br />
Inpatient, then outpatient<br />
2–4 weeks<br />
Bone or joint<br />
No residual infected tissue (e.g. postamputation)<br />
Parenteral or oral<br />
Oral or initial parenteral administration<br />
depending on the clinical situation<br />
2–5 days<br />
Residual infected soft tissue but not bone Parenteral or oral Oral or initial parenteral administration<br />
depending on the clinical situation<br />
1–3 weeks<br />
Residual infected (but viable) bone<br />
Initial parenteral, then consider oral<br />
switch<br />
Oral or initial parenteral administration<br />
depending on the clinical situation<br />
4–6 weeks<br />
No surgery, or residual dead bone<br />
postoperatively<br />
Initial parenteral, then consider oral<br />
switch<br />
Oral or initial parenteral administration<br />
depending on the clinical situation<br />
≥3 months<br />
2016). Furthermore, NICE (2016) recommends<br />
that the choice of antibiotic treatment may<br />
be influenced by the care setting, patient<br />
preferences, the clinical situation and the<br />
patient’s medical history.<br />
The IWGDF and NICE make specific<br />
recommendations with regards to antimicrobial<br />
therapy for DFIs dependent on severity (Table 2):<br />
l For mild infections, initially offer oral<br />
antibiotics with activity against Gram-positive<br />
organisms.<br />
l A 1–2-week course of antibiotic therapy is<br />
usually sufficient for mild infections.<br />
l For moderate and severe infections, administer<br />
antibiotics with activity against Gram-positive<br />
and Gram-negative organisms, including<br />
anaerobic bacteria.<br />
l For moderate infections, offer oral or initial<br />
parental administration depending on the<br />
clinical situation and choice of antibiotic.<br />
l For severe infections, administer parental<br />
therapy with a switch to oral therapy based<br />
on the clinical situation and response to<br />
treatment.<br />
l For DFO, offer 6 weeks of antibiotic therapy<br />
for patients who do not undergo surgical<br />
resection of the infected bone, according to<br />
local protocols.<br />
l For those who have had surgical intervention<br />
and all infected bone is resected, offer no more<br />
than 1 week of antibiotic therapy (Lipsky et al,<br />
2016; NICE, 2016).<br />
All open wounds are colonised with potential<br />
pathogens, but all may not be infected<br />
(Schaper et al, 2016). Lipsky et al (2012) do<br />
not recommend the prophylactic treatment of<br />
clinically uninfected wounds with antimicrobial<br />
therapy, and they advise against the selection of<br />
any specific type of dressing for DFIs with the<br />
aim of preventing an infection or improving its<br />
outcome.<br />
Outpatient or inpatient?<br />
Diabetic foot clinics typically operate through<br />
an outpatient system. The management and<br />
outcome for a DFI is superior if there is the<br />
involvement of a multidisciplinary team<br />
(MDT) that includes podiatrists, nurses,<br />
endocrinologists, infectious disease specialists<br />
and vascular and orthopaedic surgeons (Lipsky<br />
et al, 2012). In 2012, the IDSA published a<br />
clinical practice guideline for the diagnosis and<br />
treatment of DFIs (Lipsky et al, 2012) and, in<br />
2015, the IWGDF produced guidelines and a<br />
global evidence-based consensus document on<br />
the management of foot problems in diabetes<br />
(Bakker et al, 2016). Both documents provide<br />
Diabetes & Primary Care Australia Vol 2 No 1 2017 27
Antimicrobial management of diabetic foot infection<br />
Page points<br />
1. The considerations of distance<br />
of travel to outpatient clinics,<br />
family and caregiver support,<br />
adherence to antibiotic<br />
treatment and offloading<br />
regimens are important in the<br />
successful treatment of an<br />
infected diabetic foot ulcer and<br />
in the decision to manage an<br />
individual as an inpatient or<br />
outpatient.<br />
2. The optimum duration of<br />
antibiotic treatment is of<br />
significant clinical importance.<br />
3. Guidelines recommend a<br />
course of antibiotic therapy of<br />
1–2 weeks for most mild and<br />
moderate infections.<br />
the treating clinician with practical guidance<br />
on which specific patients require, and would<br />
benefit most from, hospitalisation. As acute<br />
hospitals are constantly under sustained pressure<br />
due to limited bed capacity, the question of<br />
continuing outpatient care or admission to<br />
hospital is of significant importance.<br />
The presence of a severe infection, as defined<br />
by IDSA criteria, requires emergency admission<br />
to hospital for parenteral antibiotics, assessment<br />
from a surgical specialist (possible need for<br />
surgical intervention to remove necrotic tissue,<br />
including infected bone, and to drain abscesses<br />
[Schaper et al, 2016]), and rapid access to the<br />
MDT. Imaging studies and diagnostic tests<br />
(e.g. MRI scanning) is readily accessible as<br />
an inpatient. Patients with moderate DFI<br />
with complicating features, such as severe<br />
peripheral arterial disease (which, if present,<br />
may need urgent revascularisation [Schaper et<br />
al, 2016]), or lack of home support or who are<br />
unable to comply with the required outpatient<br />
treatment regimen for psychological or social<br />
reasons should be hospitalised initially (Lipsky<br />
et al, 2012). Other factors that would result in<br />
hospitalisation include haemodynamic and<br />
metabolic stabilisation, the need for careful<br />
continuous observation, and the use of complex<br />
dressings. If intravenous therapy is required,<br />
but not available as an outpatient, or if surgical<br />
procedures (more than minor) are required, then<br />
inpatient care is optimal (Bakker et al, 2015).<br />
If an MDT is not available in the outpatient<br />
setting, people with moderate grade diabetic<br />
foot ulceration may benefit from an inpatient<br />
“short stay” of approximately 5 days to obtain<br />
diagnostic tests, and clinical consultations<br />
from the MDT. This approach addresses<br />
the complexities of the person with a DFI<br />
and allows a more complete evaluation and<br />
establishment of a treatment regimen. This<br />
clinical care pathway would allow parenteral<br />
antibiotics to be administered initially with<br />
a switch to oral agents when the patient<br />
is systemically well and culture results are<br />
available. Surgical intervention may be<br />
performed and glycaemic control would be<br />
optimised with an effective individualised<br />
discharge plan instituted.<br />
A significant factor is the social circumstances<br />
of a patient. The considerations of distance of<br />
travel to outpatient clinics, family and caregiver<br />
support, adherence to antibiotic treatment<br />
and offloading regimens are important in the<br />
successful treatment of an infected DFU and<br />
in the decision to manage an individual as an<br />
inpatient or outpatient. This highlights the need<br />
for, and the importance of, an individualised<br />
treatment plan.<br />
How long is long enough?<br />
The optimum duration of antibiotic treatment<br />
is of significant clinical importance, as<br />
under-treatment will lead to persistence of<br />
infection with inherent risk of amputation<br />
and systemic sepsis, while over-treatment<br />
may increase the risk of multi-drug-resistant<br />
organisms and antibiotic-associated infections<br />
(e.g. Clostridium difficile). The duration of<br />
antibiotic therapy for a DFI should be based<br />
on the severity of the infection, the presence or<br />
absence of bone infection, the likely or proven<br />
causative agents and the clinical response to<br />
therapy (Lipsky et al, 2012; Bakker et al, 2015).<br />
Antibiotics can usually be discontinued once the<br />
clinical signs and symptoms of infection have<br />
resolved. There is no evidence-base to support<br />
continuing antibiotic therapy until the wound<br />
is healed in order to either accelerate closure<br />
or prevent subsequent infection (Lipsky et al,<br />
2012).<br />
Guidelines recommend a course of antibiotic<br />
therapy of 1–2 weeks for most mild and<br />
moderate infections (Lipsky et al, 2012).<br />
Parenteral therapy should be administered<br />
initially for severe infections and some moderate<br />
infections, with a switch to oral therapy when<br />
the infection is responding (Bakker et al, 2015).<br />
The duration of antibiotics in moderate to severe<br />
diabetic foot infection is 1–4 weeks (Table 2).<br />
In non-healing diabetic foot ulceration<br />
(>4 weeks), the presence of underlying DFO<br />
and peripheral arterial disease should be<br />
assessed. If DFO is present, there are medical or<br />
surgical treatment approaches. Both treatment<br />
approaches have demonstrated efficacy in<br />
selected patients. Antibiotic treatment alone for<br />
DFO will likely succeed with smaller ulcers,<br />
28 Diabetes & Primary Care Australia Vol 2 No 1 2017
Antimicrobial management of diabetic foot infection<br />
with more extensive ulceration requiring<br />
surgery. Resection of infected bone will treat<br />
osteomyelitis but increases the risk of altered<br />
foot biomechanics and transfer ulceration.<br />
Surgical intervention should be considered in<br />
cases of osteomyelitis accompanied by: spreading<br />
soft tissue infection; destroyed soft tissue<br />
envelope; progressive bone destruction on X-ray;<br />
or bone protruding through the ulcer (Bakker<br />
et al, 2015). The decision to institute either a<br />
primary medical or surgical approach based on<br />
randomised clinical trial data is difficult, as the<br />
diagnosis of osteomyelitis in some trials was not<br />
based on bone culture and histology.<br />
The IWGDF guideline recommends 6 weeks<br />
of antibiotic therapy for patients who do not<br />
undergo resection of infected bone and no<br />
more than a week of antibiotic treatment if all<br />
infected bone is resected. Similar guidance<br />
is provided with the IDSA guidelines, which<br />
advise 4–6 weeks of antibiotics if there is<br />
residual infected, but viable, bone. However,<br />
the IDSA guidelines recommend greater than<br />
3 months, of antibiotic therapy if there is no<br />
surgery or residual dead bone postoperatively<br />
(Table 2 [Lipsky et al, 2012; Bakker et al,<br />
2015). There is variance in the recommendation<br />
between the two guidelines, and the decision<br />
to extend antibiotic treatment beyond 6 weeks<br />
to 3 months should be made in consultation<br />
with an infectious diseases or clinical<br />
microbiological specialist.<br />
The optimal treatment of DFI with DFO will<br />
be based on clinical severity, likely or proven<br />
causative agents and clinical progression. A<br />
consultation with an infectious diseases or<br />
clinical microbiological specialist and the wider<br />
MDT is recommended.<br />
Conclusion<br />
DFIs are a common and serious complication<br />
of diabetes (present in up to 60% of DFUs;<br />
Markakis et al, 2016). DFIs can spread rapidly<br />
to underlying tissues, including bone, and<br />
DFO is a frequent outcome. Early diagnosis<br />
of DFI and rapid initiation of antimicrobial<br />
therapy is vital to minimise the adverse patient<br />
outcomes associated with foot infections, such as<br />
amputation. The Australian guidelines, IDSA,<br />
IWGDF and NICE all offer clear guidance on<br />
appropriate antimicrobial therapy dependent on<br />
the severity of the infection, clinical situation<br />
and efficacy of the agents. <br />
n<br />
Acknowledgement<br />
This article has been modified from one<br />
previously published in The Diabetic Foot Journal<br />
(2016, 3: 132–7).<br />
Akkus G, Evran M, Gungor D et al (2016) Tinea pedis and<br />
onychomycosis frequency in diabetes mellitus patients and<br />
diabetic foot ulcers: A cross sectional-observational study.<br />
Pak J Med Sci 32: 891–5<br />
Baker IDI (2011) National Evidence-Based Guideline on<br />
Prevention, Identification and Management of Foot<br />
Complications in Diabetes (Part of the Guidelines on<br />
Management of Type 2 Diabetes) 2011. Australian<br />
Government National Health and Medical Research Council,<br />
Melbourne, Vic<br />
Bakker K, Apelqvist J, Lipsky BA et al (2016) The 2015 IWGDF<br />
guidance documents on prevention and management of foot<br />
problems in diabetes: development of an evidence-based<br />
global consensus. Diabetes Metab Res Rev 32(Suppl 1): 2–6<br />
Bravo-Molina A, Linares-Palomino JP, Lozano-Alonso S et<br />
al (2016) Influence of wound scores and microbiology on<br />
the outcome of the diabetic foot syndrome. J Diabetes<br />
Complications 30: 329–34<br />
International Diabetes Federation (2015) IDF Atlas (7 th edition).<br />
IDF, Brussels, Belgium<br />
Lipsky BA, Berendt AR, Cornia PB et al (2012) Infectious<br />
Diseases Society of America Clinical Practice Guideline for<br />
the Diagnosis and Treatment of Diabetic Foot Infections. Clin<br />
Infect Dis 54: 132–73<br />
Lipsky BA, Aragon-Sanchez J, Diggle M et al (2016) IWGDF<br />
guidance on the diagnosis and management of foot<br />
infections in persons with diabetes. Diabetes Metab Res Rev<br />
32(Suppl 1): 45–74<br />
Markakis K, Bowling FL, Boulton AJ (2016) The diabetic foot<br />
in 2015: an overview. Diabetes Metab Res Rev 32(Suppl 1):<br />
169–78<br />
NICE (2016) Diabetes in Adults NICE Quality Standards (QS6).<br />
NICE, London, UK. Available at: https://www.nice.org.uk/<br />
guidance/QS6 (accessed 20.08.2016)<br />
Peters EJ (2016) Pitfalls in diagnosing diabetic foot infections.<br />
Diabetes Metab Res Rev 32(Supp 1): 254–60<br />
Schaper NC, Van Netten JJ, Apelqvist J et al (2016) Prevention<br />
and management of foot problems in diabetes: a Summary<br />
Guidance for Daily Practice 2015, based on the IWGDF<br />
Guidance Documents. Diabetes Metab Res Rev 32(Suppl 1):<br />
7–15<br />
“Early diagnosis of<br />
diabetic foot infections<br />
and rapid initiation of<br />
antimicrobial therapy<br />
is vital to minimise<br />
the adverse patient<br />
outcomes associated<br />
with foot infections,<br />
such as amputation.”<br />
Diabetes & Primary Care Australia Vol 2 No 1 2017 29
Article<br />
What primary care clinicians should know<br />
about fenofibrate for people with diabetes<br />
Alicia J Jenkins, Andrzej S Januszewski, Emma S Scott, Anthony C Keech<br />
Citation: Jenkins AJ, Januszewski AS,<br />
Scott ES, Keech AC (2017) What<br />
primary care clinicians should know<br />
about fenofibrate for people with<br />
diabetes. Diabetes & Primary Care<br />
Australia 2: 30–4<br />
Article points<br />
1. Major clinical trials have<br />
demonstrated protective<br />
effects of oral fenofibrate<br />
against diabetic retinopathy<br />
(DR), nephropathy and some<br />
cardiovascular events in<br />
people with type 2 diabetes.<br />
2. There are multiple mechanisms<br />
by which fenofibrate may<br />
protect against the vascular<br />
and neurologic complications<br />
of diabetes, including<br />
molecular and cell-signalling<br />
effects, modulation of lipids,<br />
effects on growth factors<br />
and anti-inflammatory and<br />
anti-oxidant properties.<br />
3. Fenofibrate has been approved<br />
by the Therapeutic Goods<br />
Administration for the<br />
secondary prevention of DR<br />
in type 2 diabetes, but does<br />
not have a specific PBS-listing<br />
for this indication. However,<br />
most people with type 2<br />
diabetes qualify under current<br />
lipid/cardiovascular disease<br />
risk criteria, exactly the same<br />
as they qualify for statins.<br />
Key words<br />
– Cardiovascular disease<br />
– Fenofibrate<br />
– Risk factors<br />
Authors<br />
See page 34 for details.<br />
It has been proven that improving modifiable vascular risk factors of diabetes has a positive<br />
effect on clinical outcomes. Statins are commonly prescribed for people with type 1 and<br />
type 2 diabetes, and their use achieves similar LDL-cholesterol lowering and cardiovascular<br />
benefit as people without diabetes. The oral drug fenofibrate is less commonly used<br />
than statins, yet has been shown to have major protective effects for people with type 2<br />
diabetes, in particular for microvascular complications. In this article, the authors briefly<br />
review fenofibrate, its mechanisms of action, proven clinical benefits in type 2 diabetes and<br />
practical aspects of its prescription.<br />
Diabetes mellitus affects approximately<br />
8% of Australians and is associated with<br />
high rates of vascular complications,<br />
including diabetic retinopathy (DR),<br />
nephropathy, neuropathy and cardiovascular<br />
disease (CVD), which are associated with high<br />
personal burden and healthcare costs. Major<br />
risk factors for diabetic complications include<br />
long diabetes duration, poor glycaemic control,<br />
hypertension, smoking, renal dysfunction,<br />
dyslipidaemia and adiposity (Pedersen and<br />
Gaede, 2003; Jenkins et al, 2004). There are<br />
proven therapies relating to modifiable risk<br />
factors that improve diabetes outcomes, though<br />
unfortunately many individuals do not meet all<br />
recommended treatment targets. Lipid drugs,<br />
in particular “statins”, are commonly prescribed<br />
for people with type 1 and type 2 diabetes, and<br />
they gain similar LDL-cholesterol (LDL-C)<br />
lowering and CVD benefit as people without<br />
diabetes (Cholesterol Treatment Trialists’<br />
[CTT] Collaborators, 2008). The triglyceride<br />
(TG)-lowering/HDL-C-elevating oral drug<br />
fenofibrate is less commonly used than statins,<br />
yet has been shown to have major protective<br />
effects for people with type 2 diabetes, in<br />
particular for microvascular complications. In<br />
this article, we briefly review fenofibrate, its<br />
mechanisms of action, proven clinical benefits<br />
in type 2 diabetes and practical aspects of its<br />
prescription.<br />
Fenofibrate<br />
Pharmacokinetics<br />
Fenofibrate, a peroxisome proliferator-activated<br />
receptor alpha (PPAR-alpha) agonist, is a oncedaily<br />
oral medication, which predominantly<br />
lowers TG and small dense LDL-C and<br />
increases HDL-C and apolipoprotein A1 levels,<br />
thus reversing a common (adverse) lipid profile<br />
in people with type 2 diabetes. The active<br />
form of fenofibrate is fenofibric acid, with<br />
activation occurring at the gut wall and by<br />
circulating esterases. Fenofibric acid’s half-life<br />
is approximately 20 hours and it is excreted<br />
predominantly renally, with approximately<br />
25% being excreted in faeces (Monthly Index<br />
of Medical Specialities [MIMS], 2015; Davis<br />
et al, 2011). Due to its predominant renal<br />
excretion, fenofibrate dosage is recommended<br />
to be reduced with significant renal impairment<br />
and is contraindicated if the estimated<br />
glomerular filtration rate (eGFR) is less than<br />
10 mL/min/1.73 m 2 (Australian Medicines<br />
30 Diabetes & Primary Care Australia Vol 2 No 1 2017
What primary care clinicians should know about fenofibrate for people with diabetes<br />
Table 1. Recommended fenofibrate dose in<br />
Australia accordingly to eGFR (creatinine<br />
clearance; MIMS, 2015).<br />
eGFR (mL/min/1.73 m 2 )<br />
Dose<br />
>60 145 mg once daily<br />
20–60 96 mg once daily<br />
10–20 48 mg once daily<br />
What primary care clinicians should know about fenofibrate for people with diabetes<br />
Table 2. Percentage reduction of vascular complications with fenofibrate<br />
relative to placebo in the FIELD and ACCORD Lipid studies.<br />
Outcomes FIELD ACCORD Lipid<br />
Primary outcomes* -11 (Keech et al, 2005)<br />
Secondary outcomes<br />
Silent myocardial infarction -16 (Burgess et al, 2010) Not available<br />
Death from cardiovascular<br />
cause † -4 (Burgess et al, 2010)<br />
Coronary revascularisation ‡ -21 (Keech et al, 2005)<br />
Stroke (fatal or non-fatal) -10 (Keech et al, 2005)<br />
Any amputation due to<br />
diabetes<br />
Microvascular outcomes<br />
-36 (Rajamani et al, 2009) Not available<br />
Retinopathy § -37 (Keech et al, 2007)<br />
Albuminuria ||<br />
-14 (Keech et al, 2005; Davis<br />
et al, 2011)<br />
First minor (only) amputation -46 (Rajamani et al, 2009) Not available<br />
-8 (ACCORD Study Group,<br />
2010a)<br />
-14 (ACCORD Study Group,<br />
2010a)<br />
-6 (ACCORD Study Group,<br />
2010a)<br />
+5 (ACCORD Study Group,<br />
2010a)<br />
-40 (ACCORD Study Group,<br />
2010b)<br />
-8 (ACCORD Study Group,<br />
2010a)<br />
*FIELD: First myocardial infarction or coronary heart disease death; ACCORD Lipid: First non-fatal<br />
myocardial infraction or stroke or death from cardiovascular causes.<br />
†<br />
FIELD: Fatal myocardial infarction; ACCORD Lipid: Death from cardiovascular cause.<br />
‡<br />
FIELD: Coronary revascularisation; ACCORD Lipid: Revascularisation or hospitalisation for congestive<br />
heart failure (together with primary outcome).<br />
§<br />
FIELD: All events, laser treatment; ACCORD Lipid: Laser treatment and/or vitrectomy and/or 3-step<br />
Early Treatment Diabetic Retinopathy (ETDR) scale progression.<br />
||<br />
FIELD: Progression (normo- to microalbuminuria or from micro- to macroalbuminuria) separately,<br />
FIELD study also reported a significant 18% increase in albuminuria regression (from macro- to<br />
microalbuminuria or from micro- to normoalbuminuria) in response to fenofibrate therapy versus<br />
placebo (Keech et al, 2005; Davis et al, 2011); ACCORD Lipid: microalbuminuria incidence (postrandomisation).<br />
<br />
P
What primary care clinicians should know about fenofibrate for people with diabetes<br />
with low rates (
What primary care clinicians should know about fenofibrate for people with diabetes<br />
“In two major<br />
randomised placebocontrolled<br />
trials,<br />
FIELD and ACCORD<br />
Lipid, oral fenofibrate<br />
has shown multiple<br />
protective effects in<br />
adults with type 2<br />
diabetes and to be very<br />
well tolerated.”<br />
retinopathy progression in type 2 diabetes. N Engl J Med 363:<br />
233–44<br />
Ansquer JC, Foucher C, Aubonnet P, Le Malicot K (2009) Fibrates<br />
and microvascular complications in diabetes–insight from the<br />
FIELD study. Curr Pharm Des 15: 537–52<br />
Australian Medicines Handbook (2015) Australian Medicines<br />
Handbook 2015. AMH Pty Ltd, Adelaide, SA. Available at:<br />
http://amhonline.amh.net.au/ (accessed 07.10.16)<br />
Burgess DC et al (2007) Abstract 3693: Effects of fenofibrate on<br />
silent myocardial infarction, hospitalization for acute coronary<br />
syndromes and amputation in type 2 diabetes: the Fenofibrate<br />
Intervention and Event Lowering in Diabetes (FIELD) study.<br />
Circulation 116(Suppl 16): II_838<br />
Monthly Index of Medical Specialities (2015) Fenofibrates.<br />
Haymarket Media Group Ltd, London, UK. Available at:<br />
http://www.mims.com.au (accessed 07.10.16)<br />
Noonan JE, Jenkins AJ, Ma JX et al (2013) An update on the<br />
molecular actions of fenofibrate and its clinical effects on<br />
diabetic retinopathy and other microvascular end points in<br />
patients with diabetes. Diabetes 62: 3968–75<br />
O’Callaghan CJ, Rong P, Goh MY (2014) National guidelines for the<br />
management of absolute cardiovascular disease risk. Med J Aust<br />
200: 454–6<br />
Papademetriou V, Lovato L, Doumas M et al (2015) Chronic kidney<br />
disease and intensive glycemic control increase cardiovascular<br />
risk in patients with type 2 diabetes. Kidney Int 87: 649–59<br />
Burgess DC, Hunt D, Li L et al (2010) Incidence and predictors of<br />
silent myocardial infarction in type 2 diabetes and the effect of<br />
fenofibrate: an analysis from the Fenofibrate Intervention and<br />
Event Lowering in Diabetes (FIELD) study. Eur Heart J 31: 92–9<br />
Pedersen O, Gaede P (2003) Intensified multifactorial intervention<br />
and cardiovascular outcome in type 2 diabetes: the Steno-2<br />
study. Metabolism 52(8 Suppl 1): 19–23<br />
Chen Y et al (2011) Mechanisms for the therapeutic effect of<br />
fenofibrate on diabetic retinopathy in type 1 diabetes models.<br />
Presented at: American Diabetes Association 71 st Scientific<br />
Sessions. San Diego, Ca, USA, June 24–28<br />
Rajamani K, Colman PG, Li LP et al (2009) Effect of fenofibrate<br />
on amputation events in people with type 2 diabetes mellitus<br />
(FIELD study): a prespecified analysis of a randomised<br />
controlled trial. Lancet 373: 1780–8<br />
Chen Y, Hu Y, Lin M et al (2013) Therapeutic effects of PPAR-alpha<br />
agonists on diabetic retinopathy in type 1 diabetes models.<br />
Diabetes 62: 261–72<br />
Rajamani K et al (2015) Abstract 18987: Fenofibrate reduces<br />
peripheral neuropathy in type 2 diabetes: the Fenofibrate<br />
Intervention and Event Lowering in Diabetes (FIELD) Study.<br />
Circulation 122(Suppl 21): A18987<br />
Cholesterol Treatment Trialists’ (CTT) Collaborators (2008) Efficacy<br />
of cholesterol-lowering therapy in 18,686 people with diabetes<br />
in 14 randomised trials of statins: a meta-analysis. Lancet 371:<br />
117–25<br />
Davis TM, Ting R, Best JD et al (2011) Effects of fenofibrate on<br />
renal function in patients with type 2 diabetes mellitus: the<br />
Fenofibrate Intervention and Event Lowering in Diabetes (FIELD)<br />
Study. Diabetologia 54: 280–90<br />
Riddle MC (2010) Effects of intensive glucose lowering in the<br />
management of patients with type 2 diabetes mellitus in the<br />
Action to Control Cardiovascular Risk in Diabetes (ACCORD)<br />
trial. Circulation 122: 844–6<br />
Robins SJ, Collins D, Wittes JT et al (2001) Relation of gemfibrozil<br />
treatment and lipid levels with major coronary events: VA-HIT: a<br />
randomized controlled trial. JAMA 285: 1585–91<br />
Authors<br />
Professor Alicia Jenkins,<br />
Dr Andrzej Januszewski, Dr Emma<br />
Scott and Professor Anthony<br />
Keech are at the NHMRC Clinical<br />
Trials Centre, University of<br />
Sydney, Camperdown, NSW.<br />
Jenkins AJ, Rowley KG, Lyons TJ et al (2004) Lipoproteins and<br />
diabetic microvascular complications. Curr Pharm Des 10:<br />
3395–418<br />
Jones PH, Davidson MH (2005) Reporting rate of rhabdomyolysis<br />
with fenofibrate + statin versus gemfibrozil + any statin.<br />
Am J Cardiol 95: 120–2<br />
Keech A, Simes RJ, Barter P et al (2005) Effects of long-term<br />
fenofibrate therapy on cardiovascular events in 9795 people<br />
with type 2 diabetes mellitus (the FIELD study): randomised<br />
controlled trial. Lancet 366: 1849–61<br />
Keech AC, Mitchell P, Summanen PA et al (2007) Effect of<br />
fenofibrate on the need for laser treatment for diabetic<br />
retinopathy (FIELD study): a randomised controlled trial. Lancet<br />
370: 1687–97<br />
Manninen V, Tenkanen L, Koskinen P et al (1992) Joint effects of<br />
serum triglyceride and LDL cholesterol and HDL cholesterol<br />
concentrations on coronary heart disease risk in the Helsinki<br />
Heart Study. Implications for treatment. Circulation 85: 37–45<br />
Scott R, O’Brien R, Fulcher G et al (2009) Effects of fenofibrate<br />
treatment on cardiovascular disease risk in 9,795 individuals<br />
with type 2 diabetes and various components of the metabolic<br />
syndrome: the Fenofibrate Intervention and Event Lowering in<br />
Diabetes (FIELD) study. Diabetes Care 32: 493–8<br />
Tenenbaum A, Motro M, Fisman EZ et al (2005) Bezafibrate for the<br />
secondary prevention of myocardial infarction in patients with<br />
metabolic syndrome. Arch Intern Med 165: 1154–60<br />
Ting RD, Keech AC, Drury PL et al (2012) Benefits and safety of<br />
long-term fenofibrate therapy in people with type 2 diabetes<br />
and renal impairment: the FIELD Study. Diabetes Care 35: 218–<br />
25<br />
Tziomalos K, Athyros VG (2006) Fenofibrate: a novel formulation<br />
(Triglide) in the treatment of lipid disorders: a review. Int J<br />
Nanomedicine 1: 129–47<br />
Vasudevan AR, Jones PH (2006) Effective use of combination lipid<br />
therapy. Curr Atheroscler Rep 8: 76–84<br />
34 Diabetes & Primary Care Australia Vol 2 No 1 2017
Article<br />
Glucagon-like peptide-1 analogues –<br />
a practical guide to initiation<br />
Ralph Audehm and Laura Dean<br />
Glucagon-like peptide-1 (GLP-1) analogues are one of the more recent additions to the<br />
type 2 diabetes armamentarium and work by mimicking the incretin system to lower<br />
glucose and increase insulin. The drug class also exerts associated non-glycaemic<br />
advantages, such as weight loss. The first GLP-1 analogue was approved for use in Australia<br />
in 2007 and has been subsidised by the Pharmaceutical Benefits Scheme since 2008, yet its<br />
use remains relatively low. This article discusses the mode of action of GLP-1 analogues,<br />
their use in Australia and how to use and initiate GLP-1 analogues in people with type 2<br />
diabetes, using case studies.<br />
Glucagon-like peptide-1 (GLP-1) is a<br />
hormone released by the gut when<br />
sensory receptors in the small intestine<br />
detect dietary glucose and other nutrients.<br />
GLP-1 forms part of the “incretin effect” – the<br />
increased secretion of insulin when the same<br />
amount of glucose is ingested orally compared<br />
to when it is administered intravenously<br />
(Willard and Sloop, 2012). The incretin system<br />
potentiates glucose-induced insulin secretion<br />
and may be responsible for up to 70% of postprandial<br />
insulin secretion (Holst et al, 2009).<br />
GLP-1 is also known to be involved in the<br />
inhibition of glucagon release (thus decreasing<br />
hepatic production of glucose and fasting blood<br />
glucose levels), delayed gastric emptying (thus<br />
decreasing post-prandial blood glucose levels)<br />
and the suppression of appetite, which, in<br />
theory, leads to decreased calorie intake (Gupta,<br />
2013). The roles of GLP-1 make it an attractive<br />
target for drug development, especially for<br />
the management of type 2 diabetes where the<br />
incretin effect is severely reduced or absent<br />
in patients (Holst et al, 2009). In addition,<br />
the half-life of endogenous GLP-1 is less than<br />
2 minutes, so creating GLP-1 analogues that<br />
can be active for longer is of great benefit.<br />
Dipeptidyl peptidase-4 (DPP-4), an<br />
endogenous enzyme also involved in the<br />
incretin system, rapidly inactivates GLP-<br />
1, leading to its short half-life (Willard and<br />
Sloop, 2012). It is now possible to artificially<br />
prolong the half-life of GLP-1 and its effect<br />
by using DPP-4 inhibitors, or “gliptins”. The<br />
first DPP-4 inhibitor to be approved by the US<br />
Food and Drug Administration was saxagliptin<br />
in 2006. The drug class is well tolerated by<br />
users, is weight neutral and has a low risk of<br />
hypoglycaemia unless used in combination with<br />
medications with a risk of hypoglycaemia, such<br />
as sulfonylureas (Prasad-Reddy and Isaacs, 2015).<br />
They are administered in tablet form and are<br />
available in a combined tablet with metformin.<br />
Studies have shown they can reduce HbA 1c<br />
by<br />
4.4–7.7 mmol/mol (0.4–0.7%; Brunton, 2014).<br />
Around the same time, GLP-1 analogues were<br />
developed to be more resistant to the actions<br />
of DPP-4 inhibitors and with a longer halflife<br />
than endogenous GLP-1. The advantages<br />
and disadvantages of using GLP-1 analogues<br />
are presented in Table 1. GLP-1 analogues<br />
have been shown to reduce HbA 1c<br />
by around<br />
10.9 mmol/mol (1%; Kenkre et al, 2013) and<br />
they can be combined with insulin as GLP-1<br />
analogues are weight negative and insulin is<br />
weight positive (Cohen et al, 2013). Devices<br />
that contain both basal insulin and a GLP-1<br />
analogue in a single injection have been<br />
Citation: Audehm R, Dean L (2017)<br />
Glucagon-like peptide-1 analogues –<br />
a practical guide to initiation.<br />
Diabetes & Primary Care Australia<br />
2: 35–9<br />
Article points<br />
1. The incretin system is used<br />
to develop pharmacological<br />
therapies for type 2 diabetes.<br />
2. The incretin mimetics,<br />
glucagon-like peptide-1 (GLP-1)<br />
analogues, represent a useful<br />
and effective tool in the<br />
management of type 2 diabetes<br />
3. GLP-1 analogues are an under<br />
utilised resource in the type 2<br />
diabetes armamentarium.<br />
Key words<br />
– Administration<br />
– Glucagon-like peptide-1<br />
analogues<br />
– Glucose-lowering medication<br />
– Incretin system<br />
Authors<br />
Ralph Audehm, Associate<br />
Professor, Department of General<br />
Practice, University of Melbourne,<br />
Vic, and Director, Primary Care<br />
Diabetes Society of Australia;<br />
Laura Dean, Course Director,<br />
Graduate Certificate in Pharmacy<br />
Practice, Faculty of Pharmacy and<br />
Pharmaceutical Sciences, Monash<br />
University, Vic.<br />
Diabetes & Primary Care Australia Vol 2 No 1 2017 35
Glucagon-like peptide-1 analogues – a practical guide to initiation<br />
approved in Europe and USA (insulin degludec/<br />
liraglutide; Greig and Scott, 2015).<br />
Table 2 gives an overview of GLP-1 analogues<br />
that are approved by the Therapeutic Goods<br />
Administration (TGA) for use in Australia:<br />
exenatide, exenatide-modified release, liraglutide<br />
Table 1. Advantages and disadvantages of glucagon-like peptide-1 (GLP-1) analogues.<br />
Advantages<br />
Significant weight loss: 3–4 kg on an intention to treat analysis (Robinson et al,<br />
2013).<br />
Decreased post-prandial excursions.<br />
Glucose-dependent manner: GLP-1 analogues do not cause hypoglycaemia<br />
unless used with other medications, such as sulfonylureas or insulin.<br />
Liraglutide has recently been shown to reduce mortality in people with type 2<br />
diabetes (Marso et al, 2016).<br />
Disadvantages<br />
Gastrointestinal effects (e.g. nausea and diarrhoea): 5–10% of people will stop a<br />
GLP-1 analogue due to nausea (Meier, 2012).<br />
Pancreatitis: There was an early signal suggesting that GLP-1 analogues may<br />
increase the risk of pancreatitis. Follow-up studies have shown that the risk for<br />
pancreatitis is no greater than the background incidence of pancreatitis in people<br />
with type 2 diabetes (Forsmark, 2016).<br />
Delivery of the dose: GLP-1 analogues must be injected.<br />
Renal insufficiency: GLP-1 analogues should not be used if eGFR<br />
Glucagon-like peptide-1 analogues – a practical guide to initiation<br />
and lixisenatide. Lixisenatide is not currently<br />
being supplied in Australia.<br />
How to initiate GLP-1 analogues<br />
Exenatide<br />
Exenatide (Byetta ® ) is available in two dose<br />
forms, 5 µg and 10 µg. Patients should initiate<br />
with 5 µg twice daily. The dose should be<br />
injected within the 60 minutes before their<br />
breakfast and evening meal. Exenatide can<br />
be administered before the lunch and evening<br />
meal, as long as the injections are at least<br />
6 hours apart. The dosage can be increased<br />
to 10 µg twice a day after 1 month at 5 µg if<br />
tolerated.<br />
Exenatide-modified release<br />
Exenatide-modified release (Bydureon ® ) is a<br />
long-acting form of exenatide. It is available<br />
in a 2 mg/mL dose and it is administered<br />
once a week on the same day. There is no dose<br />
titration required and it can be given at any<br />
time of the day without reference to meals.<br />
The formulation uses microspheres to provide<br />
the modified-release properties, which results<br />
in a delayed onset of action, such that it may<br />
take 2 weeks for the exenatide to begin being<br />
released, and up to 7 weeks for complete release<br />
(Cai et al, 2013). To manage expectations,<br />
people using once-weekly exenatide should be<br />
made aware of this delay.<br />
If a patient wishes to change the day on<br />
which they administer the injection, they<br />
may do so as long as there is more than one<br />
clear day between the injections. Similarly<br />
if a patient forgets to inject the exenatidemodified<br />
release, they may administer it as<br />
soon as they remember. However, if there are<br />
only one or two days until the next scheduled<br />
dose, they should wait until the scheduled<br />
day to take their dose (Eli Lilly Australia Pty,<br />
2013).<br />
It is important to be aware that the<br />
microspheres in long-acting exenatide can lead<br />
to an inflammatory reaction and “nodules”<br />
or subcutaneous lumps developing at the<br />
injection site, which are usually asymptomatic<br />
and resolve over 4–8 weeks (DeYoung et al,<br />
2011).<br />
Liraglutide<br />
Liraglutide (Victoza ® ) is available is a single<br />
pen device containing three different dosing<br />
options: 0.6 mg, 1.2 mg and 1.8 mg. The<br />
starting dose is 0.6 mg daily, which can be<br />
increased to 1.2 mg after at least 1 week and,<br />
if tolerated, can be increased to 1.8 mg to<br />
achieve maximum efficacy. The dose is given<br />
independently of meals but should be given<br />
around the same time each day.<br />
If patients are on insulin or a sulfonylurea, it<br />
is useful to ask them to monitor their glucose<br />
levels four times per day for the first 3–4 days to<br />
identify any risk of hypoglycaemia. If the HbA 1c<br />
for a patient is
Glucagon-like peptide-1 analogues – a practical guide to initiation<br />
“It is beneficial to<br />
warn all people using<br />
glucagon-like peptide-1<br />
analogues to expect<br />
nausea, especially in<br />
the first 2–3 days after<br />
initiation.”<br />
After discussing options for treatment<br />
intensification, he elected to trial exenatide.<br />
Exenatide 5 µg was commenced twice daily and<br />
increased to 10 µg after a month. He tolerated<br />
the injection very well and found the injections<br />
easy to administer. Six months later, Mr GL had<br />
achieved a weight loss of 4 kg and his HbA 1c<br />
was<br />
40 mmol/mol (5.8%).<br />
Case 2<br />
Ms PI is a 54-year-old lady with a HbA 1c<br />
88 mol/mol (10.2%). Her blood pressure<br />
(BP) is 140/85 mmHg and she has a BMI of<br />
31 kg/m 2 . She has no nephropathy and her<br />
estimated glomerular filtration rate (eGFR) is<br />
>90 mL/min/1.73 m 2 . She has retinopathy and<br />
has had laser therapy treatment in the past. She<br />
is currently on a combined tablet of metformin<br />
and DPP-4 inhibitor (metformin 1000 g/<br />
linagliptin 2.5 mg) twice daily, but she has an<br />
erratic lifestyle and has difficulties remembering<br />
her medications. After discussing her options,<br />
including insulin, she elected to start exenatidemodified<br />
release, feeling that a weekly injection<br />
would be easier to manage. She understood<br />
that she would need insulin in the future but<br />
the GLP-1 analogue would help reduce the<br />
amount of weight she would gain with insulin.<br />
The metformin/DPP-4 inhibitor combination<br />
was stopped and the exenatide-modified release<br />
commenced with metformin XR 1000 mg twice<br />
daily. The initial injection was supervised with<br />
the practice nurse and Ms PI felt confident with<br />
the injections.<br />
At 3 weeks, she had lost 2.5 kg and her daily<br />
blood glucose levels were now in the low teens.<br />
Ms PI has had minimal side effects as a result of<br />
the change in drug regimen. She was happy with<br />
the weight loss she has achieved and improved<br />
blood glucose levels and is now more engaged<br />
with the practice and attending appointments<br />
more regularly, offering the opportunity for<br />
ongoing treatment intensification if required.<br />
Case 3<br />
RA is a 60-year-old male. He is on insulin<br />
glargine 48 units per day, metformin<br />
2000 mg daily and gliclazide MR 120 mg daily,<br />
as well as anti-hypertensives and a statin. His<br />
HbA 1c<br />
is 66 mmol/mol (8.2%). He has minimal<br />
non-proliferative retinopathy and a marginally<br />
elevated albumin–creatinine ratio (7 mg/mmol).<br />
His BP at his most recent appointment was<br />
138/70 mmHg and his BMI was 35 kg/m 2 . On<br />
discussion with the clinician, self-blood glucose<br />
monitoring results showed large post-prandial<br />
elevations: these were highest after dinner<br />
(around 12–13 mmol/L) but also high at lunch.<br />
His fasting blood glucose measurements were<br />
around 7 mmol/L. Exenatide was commenced<br />
at 5 µg bd, prior to breakfast and dinner.<br />
The device and the injection technique were<br />
demonstrated at an appointment later in the<br />
day so that he could then go home and have<br />
his evening meal. He was told to monitor his<br />
blood glucose levels four times a day and to<br />
carry jellybeans or another quick-acting glucose<br />
source with him at all times.<br />
At review 1 week later, his fasting blood<br />
glucose had dropped, and during the preceding<br />
week, were often below 5 mmol/L but not<br />
below 4 mmol/L. He was comfortable with<br />
adminstering the injections and elected to<br />
reduce his gliclazide to minimise his risk of<br />
hypoglycaemia. Another review was organised<br />
for a week’s time. If exentaide 5 µg dose<br />
continues to be well tolerated, after 1 month,<br />
the exenatide dose will be increased to 10 µg bd.<br />
How to manage the side effects of<br />
GLP-1 analogues<br />
The most common side effects reported by<br />
people using GLP-1 analogues are nausea<br />
and vomiting (Garber, 2011). Therefore, it is<br />
beneficial to warn all patients to expect nausea,<br />
especially in the first 2–3 days. The nausea will<br />
usually settle, but to diminish these effects<br />
you may suggest that they eat smaller meals or<br />
snacks in the interim. Anti-emetics can be used<br />
in the short term to help patients overcome this<br />
period of nausea. Exenatide-modified release<br />
and liraglutide have lower rates of nausea than<br />
exenatide (Garber, 2011).<br />
Summary<br />
The GLP-1 analogue drug class offers a drug<br />
regimen for people with type 2 diabetes that<br />
is well tolerated by users, weight neutral and<br />
38 Diabetes & Primary Care Australia Vol 2 No 1 2017
Glucagon-like peptide-1 analogues – a practical guide to initiation<br />
with a low risk of hypoglycaemia. It is straightforward<br />
to initiate and titrate, but it is currently<br />
underutilised. In 2015, there were just over<br />
200 000 scripts for exenatide compared to more<br />
than 2 million scripts for a DPP-4 inhibitor<br />
(The Pharmaceutical Benefits Scheme, 2015).<br />
GLP-1 analogues are an excellent addition to<br />
our armamentarium.<br />
n<br />
Declaration<br />
Ralph Audehm has received monies from Sanofi,<br />
AstraZeneca, NovoNordisk, Novartis and Lilly<br />
for consultancy work.<br />
<br />
Brunton S (2014) GLP-1 receptor agonists vs. DPP-4 inhibitors for<br />
type 2 diabetes: is one approach more successful or preferable<br />
than the other? Int J Clin Pract 68: 557–67<br />
Cai Y, Wei L, Ma L et al (2013) Long-acting preparations of<br />
exenatide. Drug Design Dev Ther 7: 963–70<br />
Cohen N, Audehm R, Pretorius E et al (2013) The rationale for<br />
combining GLP-1 receptor agonists with basal insulin. Medical<br />
J Austr 199: 246–9<br />
DeYoung MB, MacConell L, Sarin V et al (2011) Encapsulation<br />
of exenatide in poly-(d l-lactide-co-glycolide) microspheres<br />
produced an investigational long-acting once-weekly<br />
formulation for type 2 diabetes. Diabetes Technol Therapeut 13:<br />
1145–54<br />
Garber AJ (2011) Long-acting glucagon-like peptide 1 receptor<br />
agonists: a review of their efficacy and tolerability. Diabetes<br />
Care 34(Suppl 2): S279–84<br />
Greig SL, Scott LJ (2015) Insulin degludec/liraglutide: A review in<br />
type 2 diabetes. Drugs 75: 1523–34<br />
Gupta V (2013) Glucagon-like peptide-1 analogues: An overview.<br />
Indian J Endocrinol Metab 17: 413–21<br />
Holst JJ, Vilsbøll T, Deacon CF (2009) The incretin system and its<br />
role in type 2 diabetes mellitus. Mol Cell Endocrinol 297: 127–<br />
36<br />
Kenkre J, Tan T, Bloom S (2013) Treating the obese diabetic. Expert<br />
Rev Clin Pharmacol 6: 171–8<br />
Marso SP, Daniels GH, Brown-Frandsen K et al (2016) Liraglutide<br />
and cardiovascular outcomes in type 2 diabetes. N Engl J Med<br />
375: 311–22<br />
Prasad-Reddy L, Isaacs D (2015) A clinical review of GLP-1<br />
receptor agonists: efficacy and safety in diabetes and beyond.<br />
Drugs Context 4: 212283<br />
Robinson LE, Holt TA, Rees K et al (2013) Effects of exenatide<br />
and liraglutide on heart rate blood pressure and body weight:<br />
systematic review and meta-analysis. BMJ Open 3: e001986<br />
The Pharmaceutical Benefits Scheme (2015) Australian Statistics on<br />
Medicines 2015. Australian Government Department of Health,<br />
Canberra, ACT. Available at: http://www.pbs.gov.au/info/<br />
statistics/asm/asm-2015 (accessed 26.10.16)<br />
“As the most<br />
common side effects<br />
reported by people<br />
using glucagonlike<br />
peptide-1<br />
analogues are nausea<br />
and vomiting, it is<br />
beneficial to warn<br />
all patients to expect<br />
nausea, especially in<br />
the first 2–3 days of<br />
use.”<br />
Eli Lilly Australia Pty (2013) Product Information BYDUREON ®<br />
(exenatide). West Ryde, NSW. Available at: https://www.tga.gov.<br />
au/sites/default/files/auspar-exenatide-130205-pi.pdf (accessed<br />
18.11.16)<br />
Forsmark CE (2016) Incretins diabetes pancreatitis and pancreatic<br />
cancer: What the GI specialist needs to know. Pancreatology 16:<br />
10–3<br />
Trujillo JM, Nuffer W, Ellis SL (2015) GLP-1 receptor agonists: a<br />
review of head-to-head clinical studies. Ther Adv Endocrinol<br />
Metab 6: 19–28<br />
Willard FS, Sloop K (2012) Physiology and emerging biochemistry<br />
of the glucagon-like peptide-1 receptor. Exp Diabetes Res 2012:<br />
470851<br />
Diabetes & Primary Care Australia Vol 2 No 1 2017 39
The PCDSA is a multidisciplinary society with the aim<br />
of supporting primary health care professionals to deliver<br />
high quality, clinically effective care in order to improve<br />
the lives of people with diabetes.<br />
The PCDSA will<br />
Share best practice in delivering quality diabetes care.<br />
Provide high-quality education tailored to health professional needs.<br />
Promote and participate in high quality research in diabetes.<br />
Disseminate up-to-date, evidence-based information to health<br />
professionals.<br />
Form partnerships and collaborate with other diabetes related,<br />
high level professional organisations committed to the care of<br />
people with diabetes.<br />
Promote co-ordinated and timely interdisciplinary care.<br />
Membership of the PCDSA is free and members get access to a quarterly<br />
online journal and continuing professional development activities. Our first<br />
annual conference will feature internationally and nationally regarded experts<br />
in the field of diabetes.<br />
To register, visit our website:<br />
www.pcdsa.com.au