Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
ultimately move the forward reaction, and the reverse reaction<br />
will not be as preferable since there is an imbalance towards<br />
the right.<br />
NO2(g) + CO(g) ⇄ NO(g) + CO2(g)<br />
13 The reaction shown above occurs inside a closed flask.<br />
What action will shift the reaction to the left?<br />
A pumping CO gas into the closed flask<br />
B raising the total pressure inside the flask<br />
C increasing the NO concentration in the flask<br />
D venting some CO2 gas from the flask<br />
NH4Cl(s) + heat ⇄ NH3(g) + HCl(g)<br />
14 What kind of change will shift the reaction above to the right<br />
to form more products?<br />
A a decrease in total pressure<br />
B an increase in the concentration of HCl<br />
C an increase in the pressure of NH3<br />
D a decrease in temperature<br />
The only gas that is found within the reaction above is<br />
ammonia (NH3). If the total pressure is reduced within the<br />
reaction, the gas will consequently expand, as it is the<br />
logical thing to happen, given the freeformed shape of<br />
gases. In order for the reaction to counter this effect, more<br />
gas should be formed. Nonetheless, since pressure is<br />
going to decrease and the gas is going to be more spread<br />
out, the reaction will move to the right to hopefully make<br />
this enough to compensate for the uneven number of<br />
moles to moles in the reaction-Product relationship.<br />
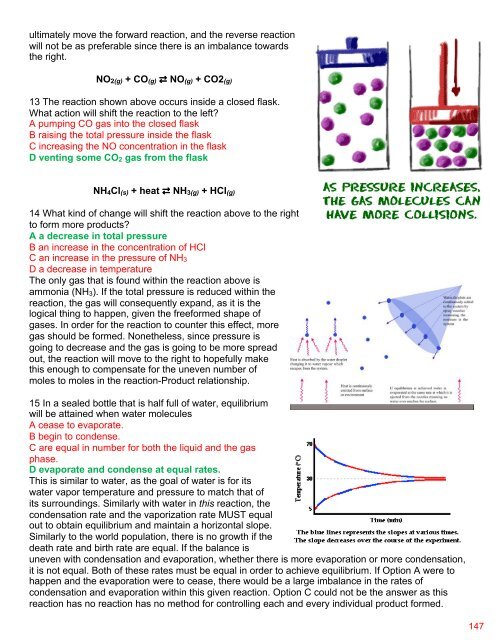
15 In a sealed bottle that is half full of water, equilibrium<br />
will be attained when water molecules<br />
A cease to evaporate.<br />
B begin to condense.<br />
C are equal in number for both the liquid and the gas<br />
phase.<br />
D evaporate and condense at equal rates.<br />
This is similar to water, as the goal of water is for its<br />
water vapor temperature and pressure to match that of<br />
its surroundings. Similarly with water in this reaction, the<br />
condensation rate and the vaporization rate MUST equal<br />
out to obtain equilibrium and maintain a horizontal slope.<br />
Similarly to the world population, there is no growth if the<br />
death rate and birth rate are equal. If the balance is<br />
uneven with condensation and evaporation, whether there is more evaporation or more condensation,<br />
it is not equal. Both of these rates must be equal in order to achieve equilibrium. If Option A were to<br />
happen and the evaporation were to cease, there would be a large imbalance in the rates of<br />
condensation and evaporation within this given reaction. Option C could not be the answer as this<br />
reaction has no reaction has no method for controlling each and every individual product formed.<br />
147