TÌM HIỂU VỀ CHUẨN ĐỘ ĐA AXIT - ĐA BAZƠ
LINK DOCS.GOOGLE: https://drive.google.com/file/d/0B_NNtKpVZTUYVzBlVnV2TUhNSUU/view?usp=sharing
LINK DOCS.GOOGLE:
https://drive.google.com/file/d/0B_NNtKpVZTUYVzBlVnV2TUhNSUU/view?usp=sharing
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
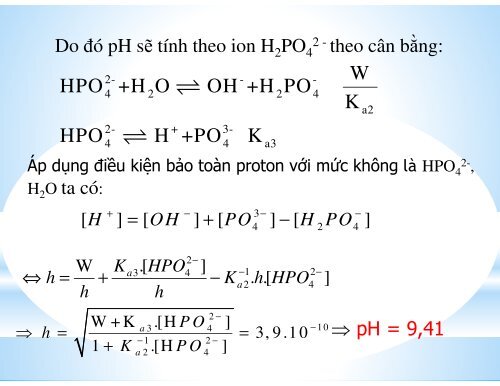
Do đó pH sẽ tính theo ion H 2 PO<br />
2 -<br />
4 theo cân bằng:<br />
W<br />
2- - -<br />
HPO<br />
4<br />
+H<br />
2O ⇌ OH +H<br />
2PO 4<br />
K<br />
a2<br />
HPO ⇌ H +PO K<br />
Áp dụng điều kiện bảo toàn proton với mức không là HPO 4<br />
2-<br />
,<br />
H 2 O ta có:<br />
2- + 3-<br />
4 4 a3<br />
[ H ] = [ OH ] + [ PO ] − [ H PO ]<br />
+ − 3 − −<br />
4 2 4<br />
2−<br />
W Ka3.[ HPO4<br />
] −1 2−<br />
⇔ h = + − Ka2. h.[ HPO4<br />
]<br />
h h<br />
⇒ W + K .[H P O ]<br />
h = 1 + K .[H ]<br />
=<br />
2 −<br />
a 3 4<br />
− 1 2 −<br />
a 2<br />
P O<br />
4<br />
3, 9 .1 0<br />
− 1 0<br />
⇒ pH = 9,41