Breakthroughs of Cancer Immunotherapy in 2017
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
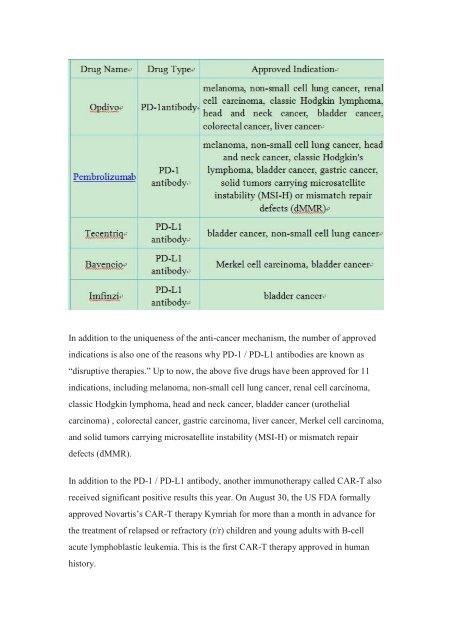
In addition to the uniqueness <strong>of</strong> the anti-cancer mechanism, the number <strong>of</strong> approved<br />
<strong>in</strong>dications is also one <strong>of</strong> the reasons why PD-1 / PD-L1 antibodies are known as<br />
“disruptive therapies.” Up to now, the above five drugs have been approved for 11<br />
<strong>in</strong>dications, <strong>in</strong>clud<strong>in</strong>g melanoma, non-small cell lung cancer, renal cell carc<strong>in</strong>oma,<br />
classic Hodgk<strong>in</strong> lymphoma, head and neck cancer, bladder cancer (urothelial<br />
carc<strong>in</strong>oma) , colorectal cancer, gastric carc<strong>in</strong>oma, liver cancer, Merkel cell carc<strong>in</strong>oma,<br />
and solid tumors carry<strong>in</strong>g microsatellite <strong>in</strong>stability (MSI-H) or mismatch repair<br />
defects (dMMR).<br />
In addition to the PD-1 / PD-L1 antibody, another immunotherapy called CAR-T also<br />
received significant positive results this year. On August 30, the US FDA formally<br />
approved Novartis’s CAR-T therapy Kymriah for more than a month <strong>in</strong> advance for<br />
the treatment <strong>of</strong> relapsed or refractory (r/r) children and young adults with B-cell<br />
acute lymphoblastic leukemia. This is the first CAR-T therapy approved <strong>in</strong> human<br />
history.