Rebirth of Water - 2017-2018
ACAP Saint John, Water Quality and Fish Communities Monitoring Report http://www.acapsj.org
ACAP Saint John, Water Quality and Fish Communities Monitoring Report
http://www.acapsj.org
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
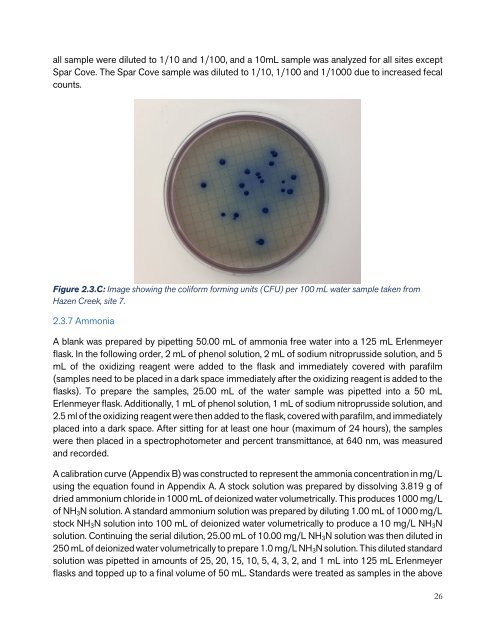
all sample were diluted to 1/10 and 1/100, and a 10mL sample was analyzed for all sites except<br />
Spar Cove. The Spar Cove sample was diluted to 1/10, 1/100 and 1/1000 due to increased fecal<br />
counts.<br />
Figure 2.3.C: Image showing the coliform forming units (CFU) per 100 mL water sample taken from<br />
Hazen Creek, site 7.<br />
2.3.7 Ammonia<br />
A blank was prepared by pipetting 50.00 mL <strong>of</strong> ammonia free water into a 125 mL Erlenmeyer<br />
flask. In the following order, 2 mL <strong>of</strong> phenol solution, 2 mL <strong>of</strong> sodium nitroprusside solution, and 5<br />
mL <strong>of</strong> the oxidizing reagent were added to the flask and immediately covered with parafilm<br />
(samples need to be placed in a dark space immediately after the oxidizing reagent is added to the<br />
flasks). To prepare the samples, 25.00 mL <strong>of</strong> the water sample was pipetted into a 50 mL<br />
Erlenmeyer flask. Additionally, 1 mL <strong>of</strong> phenol solution, 1 mL <strong>of</strong> sodium nitroprusside solution, and<br />
2.5 ml <strong>of</strong> the oxidizing reagent were then added to the flask, covered with parafilm, and immediately<br />
placed into a dark space. After sitting for at least one hour (maximum <strong>of</strong> 24 hours), the samples<br />
were then placed in a spectrophotometer and percent transmittance, at 640 nm, was measured<br />
and recorded.<br />
A calibration curve (Appendix B) was constructed to represent the ammonia concentration in mg/L<br />
using the equation found in Appendix A. A stock solution was prepared by dissolving 3.819 g <strong>of</strong><br />
dried ammonium chloride in 1000 mL <strong>of</strong> deionized water volumetrically. This produces 1000 mg/L<br />
<strong>of</strong> NH 3 N solution. A standard ammonium solution was prepared by diluting 1.00 mL <strong>of</strong> 1000 mg/L<br />
stock NH 3 N solution into 100 mL <strong>of</strong> deionized water volumetrically to produce a 10 mg/L NH 3 N<br />
solution. Continuing the serial dilution, 25.00 mL <strong>of</strong> 10.00 mg/L NH 3 N solution was then diluted in<br />
250 mL <strong>of</strong> deionized water volumetrically to prepare 1.0 mg/L NH 3 N solution. This diluted standard<br />
solution was pipetted in amounts <strong>of</strong> 25, 20, 15, 10, 5, 4, 3, 2, and 1 mL into 125 mL Erlenmeyer<br />
flasks and topped up to a final volume <strong>of</strong> 50 mL. Standards were treated as samples in the above<br />
26