Foreign Cooperating Institutions - Institute of Inorganic Chemistry ...
Foreign Cooperating Institutions - Institute of Inorganic Chemistry ...
Foreign Cooperating Institutions - Institute of Inorganic Chemistry ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
A model study <strong>of</strong> dickite intercalated with formamide<br />
and N-methylformamide<br />
E. Scholtzová, Ľ. Benco, D. Tunega<br />
The study <strong>of</strong> bonding <strong>of</strong> small molecules within the interlayer space <strong>of</strong> clay minerals<br />
enhances the understanding <strong>of</strong> the intercalation process and formation <strong>of</strong> contacts between<br />
intercalated molecule and host matrix. Local geometry and orientation <strong>of</strong> intercalated<br />
molecules <strong>of</strong> formamide (FA) and N-methylformamide (NMFA) in the clay mineral dickite<br />
(D) was studied by means <strong>of</strong> Density Functional Theory (DFT) calculations. Ten<br />
configurations with different orientation <strong>of</strong> the intercalated molecule were investigated for<br />
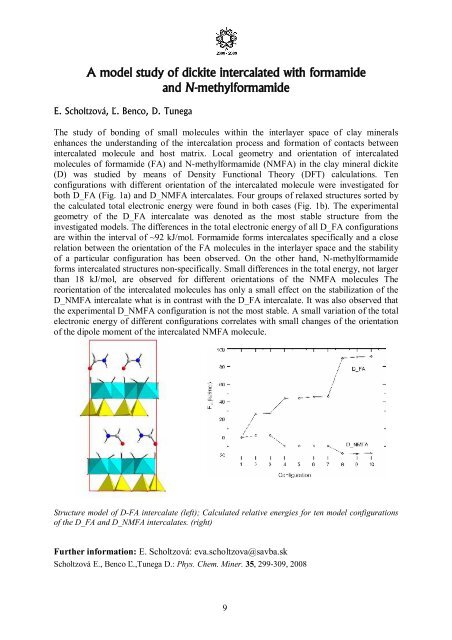
both D_FA (Fig. 1a) and D_NMFA intercalates. Four groups <strong>of</strong> relaxed structures sorted by<br />
the calculated total electronic energy were found in both cases (Fig. 1b). The experimental<br />
geometry <strong>of</strong> the D_FA intercalate was denoted as the most stable structure from the<br />
investigated models. The differences in the total electronic energy <strong>of</strong> all D_FA configurations<br />
are within the interval <strong>of</strong> ~92 kJ/mol. Formamide forms intercalates specifically and a close<br />
relation between the orientation <strong>of</strong> the FA molecules in the interlayer space and the stability<br />
<strong>of</strong> a particular configuration has been observed. On the other hand, N-methylformamide<br />
forms intercalated structures non-specifically. Small differences in the total energy, not larger<br />
than 18 kJ/mol, are observed for different orientations <strong>of</strong> the NMFA molecules The<br />
reorientation <strong>of</strong> the intercalated molecules has only a small effect on the stabilization <strong>of</strong> the<br />
D_NMFA intercalate what is in contrast with the D_FA intercalate. It was also observed that<br />
the experimental D_NMFA configuration is not the most stable. A small variation <strong>of</strong> the total<br />
electronic energy <strong>of</strong> different configurations correlates with small changes <strong>of</strong> the orientation<br />
<strong>of</strong> the dipole moment <strong>of</strong> the intercalated NMFA molecule.<br />
Structure model <strong>of</strong> D-FA intercalate (left); Calculated relative energies for ten model configurations<br />
<strong>of</strong> the D_FA and D_NMFA intercalates. (right)<br />
Further information: E. Scholtzová: eva.scholtzova@savba.sk<br />
Scholtzová E., Benco Ľ.,Tunega D.: Phys. Chem. Miner. 35, 299-309, 2008<br />
9