Foreign Cooperating Institutions - Institute of Inorganic Chemistry ...
Foreign Cooperating Institutions - Institute of Inorganic Chemistry ...
Foreign Cooperating Institutions - Institute of Inorganic Chemistry ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Organoclays and their applications<br />
J. Hrachová, Ľ. Jankovič, P. Komadel, J. Madejová, H. Pálková<br />
Montmorillonites (MMT), the main minerals in bentonites belonging to smectite group, are<br />
<strong>of</strong>ten used in many industrial applications, either in their natural form or after appropriate<br />
modification. Natural smectites with inorganic exchangeable cations, e.g. Ca 2+ or Na + , and<br />
their high affinity to water gives rise to hydrophilic character <strong>of</strong> smectite surface.<br />
Replacement <strong>of</strong> inorganic by organic (alkylammonium) cations changes the clay mineral<br />
surfaces to more hydrophobic. Final properties <strong>of</strong> organo-modified MMTs (organoclays)<br />
depend on their layer charge, the type <strong>of</strong> the organic cation used; the cation/clay mineral ratio,<br />
etc. For such materials higher stability upon mechanochemical or acid treatment was observed<br />
[1,2]. Montmorillonites in their natural forms are considered ineffective adsorbents <strong>of</strong> nonpolar<br />
organic compounds polluting frequently surface water because these compounds cannot<br />
effectively compete with highly polar water for adsorption sites on smectite surfaces.<br />
However, after exchange with organic cations with long alkyl chains adsorption on the<br />
smectites has been impressive. On the other side, smectite<br />
modified with small alkylammonium cations did not display<br />
much better adsorption abilities compared to their natural<br />
forms. Improvement was achieved only for low charged<br />
smectites. Nowadays, montmorillonite is the most widely used<br />
clay mineral as nan<strong>of</strong>iller because <strong>of</strong> its cation-exchange<br />
capacity and large active surface area when sufficiently<br />
delaminated. The layer thickness is ~1 nm, while the lateral<br />
dimensions <strong>of</strong> the layers vary up to several microns or even<br />
more, i.e. at least one dimension <strong>of</strong> the filler is in the<br />
nanometer range. Of particular interest is recently developed<br />
nanocomposite technology consisting <strong>of</strong> interactions <strong>of</strong> a<br />
polymer with an organoclay [3] Alkylammonium or<br />
alkylphosphonium cations in the organoclays lead to a<br />
decrease <strong>of</strong> the surface energy <strong>of</strong> the inorganic host and<br />
improve the wetting <strong>of</strong> the filler surface by polymer matrix,<br />
resulting in larger interlayer spacing. Additionally, these<br />
cations can provide functional groups that may react with the<br />
polymer matrix, or even initiate the polymerization <strong>of</strong><br />
monomers to improve interactions on the interface between<br />
the inorganic and the polymer matrix and to advance beneficial<br />
properties <strong>of</strong> nanocomposites.<br />
10<br />
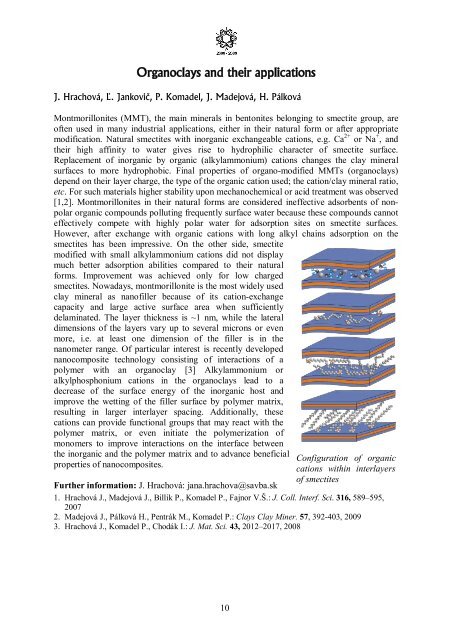
Configuration <strong>of</strong> organic<br />
cations within interlayers<br />
<strong>of</strong> smectites<br />
Further information: J. Hrachová: jana.hrachova@savba.sk<br />
1. Hrachová J., Madejová J., Billik P., Komadel P., Fajnor V.Š.: J. Coll. Interf. Sci. 316, 589–595,<br />
2007<br />
2. Madejová J., Pálková H., Pentrák M., Komadel P.: Clays Clay Miner. 57, 392-403, 2009<br />
3. Hrachová J., Komadel P., Chodák I.: J. Mat. Sci. 43, 2012–2017, 2008