March 3 - 5,1999, Karlsruhe, Germany - FZK
March 3 - 5,1999, Karlsruhe, Germany - FZK
March 3 - 5,1999, Karlsruhe, Germany - FZK
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
the real ones [24], but the more advanced mixing<br />
rules and the regression of their parameters also<br />
contribute greatly to this considerable accuracy.<br />
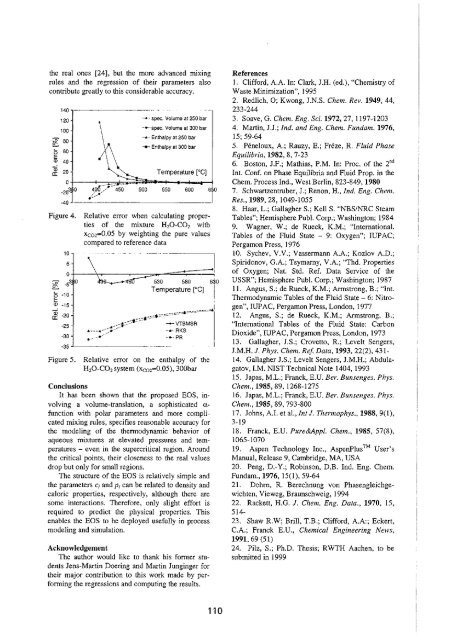
Figure 4.<br />
10<br />
0 -10-<br />
® -15-<br />
1 -20 H<br />
-25 -<br />
-30-<br />
-35<br />
spec. Volume at 250 bar<br />
*•• spec. Volume at 300 bar<br />
•*- Enthalpy at 250 bar<br />
*- Enthalpy at 300 bar<br />
Relative error when calculating properties<br />
of the mixture H 20-C0 2 with<br />
Xco2=0.05 by weighting the pure values<br />
compared to reference data<br />
i*0 530 580<br />
Temperature [°C]<br />
-•-VTBMSR<br />
-+-RKS<br />
Figure 5. Relative error on the enthalpy of the<br />
Ff 20-C0 2 system (x CO2=0.05), 300bar<br />
Conclusions<br />
It has been shown that the proposed EOS, involving<br />
a volume-translation, a sophisticated afunction<br />
with polar parameters and more complicated<br />
mixing rules, specifies reasonable accuracy for<br />
the modeling of the thermodynamic behavior of<br />
aqueous mixtures at elevated pressures and temperatures<br />
- even in the supercritical region. Around<br />
the critical points, their closeness to the real values<br />
drop but only for small regions.<br />
The structure of the EOS is relatively simple and<br />
the parameters c,- and p-, can be related to density and<br />
caloric properties, respectively, although there are<br />
some interactions. Therefore, only slight effort is<br />
required to predict the physical properties. This<br />
enables the EOS to be deployed usefully in process<br />
modeling and simulation.<br />
Acknowledgement<br />
The author would like to thank his former students<br />
Jens-Martin Doering and Martin Junginger for<br />
their major contribution to this work made by performing<br />
the regressions and computing the results.<br />
PR<br />
650<br />
630<br />
110<br />
References<br />
1. Clifford, A.A. In: Clark, J.H. (ed.), "Chemistry of<br />
Waste Minimization", 1995<br />
2. Redlich, O; Kwong, J.N.S. Chem. Rev. 1949, 44,<br />
233-244<br />
3. Soave, G. Chem. Eng. Sei. 1972, 27, 1197-1203<br />
4. Martin, J.J.; Ind. and Eng. Chem. Fundam. 1976,<br />
15; 59-64<br />
5. Peneloux, A.; Rauzy, E.; Freze, R. Fluid Phase<br />
Equilibria, 1982, 8,7-23<br />
6. Boston, J.F.; Mathias, P.M. In: Proc. of the 2 nd<br />
Int. Conf. on Phase Equilibria and Fluid Prop, in the<br />
Chem. Process Ind., West Berlin, 823-849,1980<br />
7. Schwartzentruber, J.; Renon, FL, Ind. Eng. Chem.<br />
Res., 1989,28, 1049-1055<br />
8. Haar, L.; Gallagher S.; Kell S. "NBS/NRC Steam<br />
Tables"; Hemisphere Publ. Corp.; Washington; 1984<br />
9. Wagner, W.; de Rueck, K.M.; "International.<br />
Tables of the Fluid State - 9: Oxygen"; IUP AC;<br />
Pergamon Press, 1976<br />
10. Sychev, V.V.; Vassermann A.A.; Kozlov A.D.;<br />
Spiridonov, G.A.; Tsymarny, V.A.; "Thd. Properties<br />
of Oxygen; Nat. Std. Ref. Data Service of the<br />
USSR"; Hemisphere Publ. Corp.; Washington; 1987<br />
11. Angus, S.; de Rueck, K.M.; Armstrong, B.; "Int.<br />
Thermodynamic Tables of the Fluid State - 6: Nitrogen",<br />
IUP AC, Pergamon Press, London, 1977<br />
12. Angus, S.; de Rueck, K.M.; Armstrong, B.;<br />
"International Tables of the Fluid State: Carbon<br />
Dioxide", IUP AC, Pergamon Press, London, 1973<br />
13. Gallagher, J.S.; Crovetto, R.; Levelt Sengers,<br />
J.M.H. J. Phys. Chem. Ref. Data, 1993, 22(2), 431-<br />
14. Gallagher J.S.; Levelt Sengers, J.M.H.; Abdulagatov,<br />
I.M. NIST Technical Note 1404, 1993<br />
15. Japas, M.L.; Franck, E.U. Ber. Bunsenges. Phys.<br />
Chem., 1985, 89, 1268-1275<br />
16. Japas, M.L.; Franck, E.U. Ber. Bunsenges. Phys.<br />
Chem., 1985, 89, 793-800<br />
17. Johns, A.I. et al., IntJ. Thermophys., 1988, 9(1),<br />
3-19<br />
18. Franck, E.U. Pure&Appl. Chem., 1985, 57(8),<br />
1065-1070<br />
19. Aspen Technology Inc., AspenPlus User's<br />
Manual, Release 9, Cambridge, MA, USA<br />
20. Peng, D.-Y.; Robinson, D.B. Ind. Eng. Chem.<br />
Fundam., 1976, 15(1), 59-64<br />
21. Dohm, R. Berechnung von Phasengleichgewichten,<br />
Vieweg, Braunschweig, 1994<br />
22. Rackett, H.G. J. Chem. Eng. Data., 1970, 15,<br />
514-<br />
23. Shaw R.W; Brill, T.B.; Clifford, A.A:; Eckert,<br />
CA.; Franck E.U., Chemical Engineering News,<br />
1991,69 (51)<br />
24. Pilz, S.; Ph.D. Thesis; RWTH Aachen, to be<br />
submitted in <strong>1999</strong>











![{A1[]Sp - Bibliothek](https://img.yumpu.com/21908054/1/184x260/a1sp-bibliothek.jpg?quality=85)




