March 3 - 5,1999, Karlsruhe, Germany - FZK
March 3 - 5,1999, Karlsruhe, Germany - FZK
March 3 - 5,1999, Karlsruhe, Germany - FZK
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
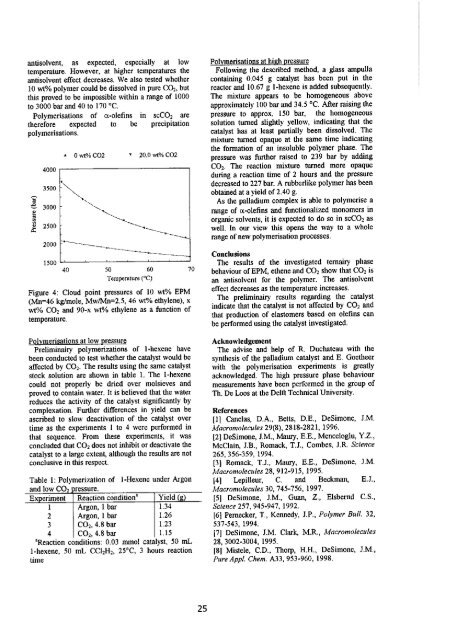
antisolvent, as expected, especially at low<br />
temperature. However, at higher temperatures the<br />
antisolvent effect decreases. We also tested whether<br />
10 wt% polymer could be dissolved in pure CQ2, but<br />
this proved to be impossible within a range of 1000<br />
to 3000 bar and 40 to 170 °C.<br />
Polymerisations of a-olefins in scC0 2 are<br />
therefore expected to be precipitation<br />
polymerisations.<br />
Pressure (bar)<br />
4000<br />
3500<br />
3000<br />
2500<br />
2000<br />
1500<br />
* 0 wt% C02 ' 20.0 wt% C02<br />
- \<br />
. \ .<br />
-1<br />
\<br />
i 1 1— 1 J 1<br />
40 50 60 70<br />
Temperature (°C)<br />
Figure 4: Cloud point pressures of 10 wt% EPM<br />
(Mn=46 kg/mole, Mw/Mn=2.5, 46 wt% ethylene), x<br />
wt% C0 2 and 90-x wt% ethylene as a function of<br />
temperature.<br />
Polymerisations at low pressure<br />
Preliminairy polymerizations of 1-hexene have<br />
been conducted to test whether the catalyst would be<br />
affected by C0 2. The results using the same catalyst<br />
stock solution are shown in table 1. The 1-hexene<br />
could not properly be dried over molsieves and<br />
proved to contain water. It is believed that the water<br />
reduces the activity of the catalyst significantly by<br />
complexation. Further differences in yield can be<br />
ascribed to slow deactivation of the catalyst over<br />
time as the experiments 1 to 4 were performed in<br />
that sequence. From these experiments, it was<br />
concluded that C0 2 does not inhibit or deactivate the<br />
catalyst to a large extent, although the results are not<br />
conclusive in this respect.<br />
Table 1: Polymerization of 1-Hexene under Argon<br />
and low C0 2 pressure.<br />
Experiment Reaction condition Yield (g)<br />
1 Argon, 1 bar 1.34<br />
2 Argon, 1 bar 1.26<br />
3 C02,4.8bar 1.23<br />
4 C02,4.8bar 1.15<br />
"Reaction conditions: 0.03 mmol catalyst, 50 mL<br />
1-hexene, 50 mL CC12H2, 25°C, 3 hours reaction<br />
time<br />
25<br />
Polymerisations at high pressure<br />
Following the described method, a glass ampulla<br />
containing 0.045 g catalyst has been put in the<br />
reactor and 10.67 g 1-hexene is added subsequently.<br />
The mixture appears to be homogeneous above<br />
approximately 100 bar and 34.5 °C. After raising the<br />
pressure to approx. 150 bar, the homogeneous<br />
solution turned slightly yellow, indicating that the<br />
catalyst has at least partially been dissolved. The<br />
mixture turned opaque at the same time indicating<br />
the formation of an insoluble polymer phase. The<br />
pressure was further raised to 239 bar by adding<br />
C0 2. The reaction mixture turned more opaque<br />
during a reaction time of 2 hours and the pressure<br />
decreased to 227 bar. A rubberlike polymer has been<br />
obtained at a yield of 2.40 g.<br />
As the palladium complex is able to polymerise a<br />
range of a-olefins and functionalized monomers in<br />
organic solvents, it is expected to do so in scC0 2 as<br />
well. In our view this opens the way to a whole<br />
range of new polymerisation processes.<br />
Conclusions<br />
The results of the investigated ternairy phase<br />
behaviour of EPM, ethene and C0 2 show that C0 2 is<br />
an antisolvent for the polymer. The antisolvent<br />
effect decreases as the temperature increases.<br />
The preliminairy results regarding the catalyst<br />
indicate that the catalyst is not affected by C0 2 and<br />
that production of elastomers based on olefins can<br />
be performed using the catalyst investigated.<br />
Acknowledgement<br />
The advise and help of R. Duchateau with the<br />
synthesis of the palladium catalyst and E. Goetheer<br />
with the polymerisation experiments is greatly<br />
acknowledged. The high pressure phase behaviour<br />
measurements have been performed in the group of<br />
Th. De Loos at the Delft Technical University.<br />
References<br />
[1] Canelas, D.A., Betts, D.E., DeSimone, J.M.<br />
Macromolecules 29(8), 2818-2821, 1996.<br />
[2] DeSimone, J.M., Maury, E.E., Menceloglu, Y.Z.,<br />
McClain, J.B., Romack, T.J., Combes, J.R. Science<br />
265, 356-359, 1994.<br />
[3] Romack, T.J., Maury, E.E., DeSimone, J.M.<br />
Macromolecules 28, 912-915, 1995.<br />
[4] Lepilleur, C. and Beckman, E.J.,<br />
Macromolecules 30, 745-756, 1997.<br />
[5] DeSimone, J.M., Guan, Z., Eisbernd C.S.,<br />
Science 257, 945-947, 1992.<br />
[6] Pernecker, T., Kennedy, J.P., Polymer Bull, 32,<br />
537-543, 1994.<br />
[7] DeSimone, J.M. Clark, M.R., Macromolecules<br />
28, 3002-3004, 1995.<br />
[8] Mistele, CD., Thorp, H.H., DeSimone, J.M.,<br />
Pure Appl. Chem. A33, 953-960, 1998.











![{A1[]Sp - Bibliothek](https://img.yumpu.com/21908054/1/184x260/a1sp-bibliothek.jpg?quality=85)




