Review: MSCs and Exosomes Production
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
www.pelobiotech.com<br />
<strong>MSCs</strong> <strong>and</strong> Extracellular Vesicle <strong>Production</strong>:<br />
A Gateway to Regenerative Medicine<br />
Mesenchymal stem / stromal cells (<strong>MSCs</strong>) have emerged as a promising tool in regenerative<br />
medicine due to their unique properties, including self-renewal <strong>and</strong> differentiation capability.<br />
One of the key mechanisms through which <strong>MSCs</strong> exert their therapeutic effects is via paracrine<br />
signalling, which includes the production <strong>and</strong> secretion of Extracellular Vesicle (EVs), often<br />
termed as exosomes or macrovesicles that play a crucial role in intercellular communication<br />
<strong>and</strong> tissue repair. This article provides an overview of <strong>MSCs</strong>, their role in EVs production, <strong>and</strong><br />
the potential applications of MSC-derived EVs in regenerative medicine. In the scientific<br />
community is a very active discussion regarding the appropriate nomenclature for<br />
mesenchymal stem cells (<strong>MSCs</strong>): Some experts suggest <strong>MSCs</strong> should be more accurately<br />
termed "stromal cells" to reflect their supportive role in tissue rather than their stem cell-like<br />
qualities 24-27 . So here we will incorporate both names <strong>and</strong> refer to these cells mesenchymal<br />
stem/stromal cells (<strong>MSCs</strong>) throughout this article.<br />
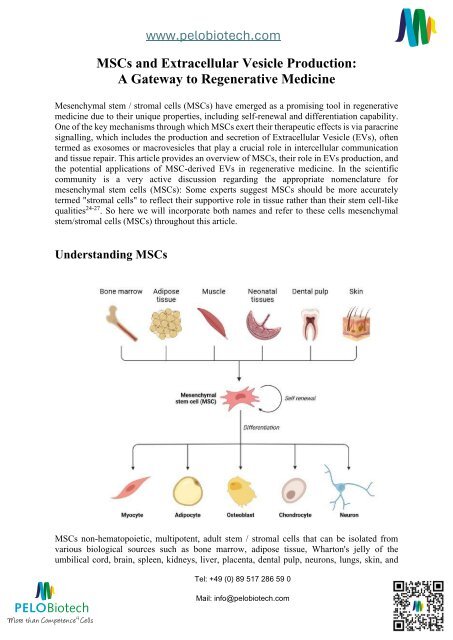
Underst<strong>and</strong>ing <strong>MSCs</strong><br />
<strong>MSCs</strong> non-hematopoietic, multipotent, adult stem / stromal cells that can be isolated from<br />
various biological sources such as bone marrow, adipose tissue, Wharton's jelly of the<br />
umbilical cord, brain, spleen, kidneys, liver, placenta, dental pulp, neurons, lungs, skin, <strong>and</strong><br />
Tel: +49 (0) 89 517 286 59 0<br />
Mail: info@pelobiotech.com
www.pelobiotech.com<br />
breast milk. <strong>MSCs</strong> have further been defined by the International Society for Cellular Therapy<br />
(ISCT) based on specific criteria outlined in a position statement from 2006. According to these<br />
criteria, <strong>MSCs</strong> must exhibit certain characteristics to be classified as such. These include the<br />
ability to adhere to plastic surfaces during in vitro culture, expression of specific surface<br />
markers such as CD105, CD73, <strong>and</strong> CD90 while lacking CD45, CD34, CD14, or CD11b,<br />
expression markers <strong>and</strong> the capacity to differentiate into osteoblasts, adipocytes, <strong>and</strong><br />
chondrocytes under specific in vitro conditions 22,23 . These criteria are crucial for the<br />
identification <strong>and</strong> st<strong>and</strong>ardization of <strong>MSCs</strong> across various tissue sources. Importantly, the<br />
tissue source of <strong>MSCs</strong> can influence their therapeutic potential, making it essential to<br />
underst<strong>and</strong> the differences between <strong>MSCs</strong> isolated from different tissues to predict their<br />
behaviour <strong>and</strong> widen their clinical use 1 .<br />
Extracellular Vesicles: Nature's Nanoscale Messengers<br />
EVs, nanoscale membrane-bound vesicles released by various cell types, including <strong>MSCs</strong>, play<br />
a vital role in an array of cellular functions, including intercellular communication, cell<br />
differentiation, <strong>and</strong> proliferation, angiogenesis, stress response, <strong>and</strong> immune signalling. The<br />
ability to carry out these different functions is because of the complexity of EVs. These vesicles<br />
carry <strong>and</strong> transfer functional cargo like proteins, messenger RNAs, microRNAs, cytokines,<br />
lipids, cell surface receptors, enzymes, <strong>and</strong> transcription factors from cells to the recipient cells.<br />
Their sizes range from 30 to 150 nanometres, originating from a specialized biogenesis<br />
pathway.<br />
The composition of EVs is contingent on the donor cell type <strong>and</strong> the physiological context of<br />
production. EVs interact with recipient cells through specific adhesion molecules <strong>and</strong> can be<br />
internalized via multiple pathways, dependent on the proximity of the target cells. The nucleic<br />
acid content in EVs is particularly influential in their functional capacity. They harbour distinct<br />
markers including tetraspanins (CD9, CD63, CD81), integrins, MHC molecules, HSP70,<br />
HSP90, Alix, TSG101, <strong>and</strong> GTPases. The lipid bilayer encapsulating EVs confers stability <strong>and</strong><br />
protection, facilitating their biological roles. <strong>MSCs</strong> release large amounts of EVs for cell-tocell<br />
communication, maintaining a dynamic <strong>and</strong> homeostatic microenvironment for tissue<br />
repair <strong>and</strong> regeneration 2,28 . Furthermore, EVs have been implicated in various physiological<br />
<strong>and</strong> pathological processes, including cardiovascular diseases <strong>and</strong> neurogenesis 3,29-31 .<br />
Tel: +49 (0) 89 517 286 59 0<br />
Mail: info@pelobiotech.com
www.pelobiotech.com<br />
MSC-Derived EVs: Key Agents in Regenerative Medicine<br />
Past research has demonstrated that, despite positive effects in various settings, <strong>MSCs</strong> were<br />
barely detected in affected tissues, resulting in the hypothesis that they mainly act via their<br />
secretome rather than in a direct cellular manner 15 . Using the examples of an acute kidney<br />
injury model <strong>and</strong> a myocardial infarction model that <strong>MSCs</strong> were found to exert their<br />
therapeutic effects EVs 33 .<br />
MSC-derived EVs have become key players in regenerative medicine, showcasing a broad<br />
range of therapeutic effects. Originating from <strong>MSCs</strong>, these EVs carry immunomodulatory,<br />
regenerative, <strong>and</strong> anti-inflammatory traits, making them highly effective in tissue repair,<br />
angiogenesis, inflammation control, <strong>and</strong> wound healing. They contribute significantly to<br />
critical cellular processes, including angiogenesis, fibrosis reduction, <strong>and</strong> remodelling of the<br />
extracellular matrix. They are particularly promising for treating a spectrum of conditions such<br />
as cardiovascular, renal, hepatic, pulmonary, <strong>and</strong> neurodegenerative diseases, <strong>and</strong> they also<br />
exhibit antimicrobial effects.<br />
Unlike <strong>MSCs</strong> themselves, which can pose challenges related to cell viability, potential for<br />
immune rejection, <strong>and</strong> the complexity of direct use in regenerative therapies, MSC-EVs offer<br />
a safer, more stable, <strong>and</strong> potentially more effective alternative. In contrast to cell therapies,<br />
EVs are not self-replicating, <strong>and</strong> they lack endogenous tumour formation potentials. EVs do<br />
not seem to sense environmental conditions, <strong>and</strong> thus, their biological activity can be predicted<br />
more reliably than that of cells. Preconditioning <strong>and</strong> engineering techniques have enhanced the<br />
efficacy of MSC-EVs, paving the way for improved outcomes in cell-free therapeutic<br />
interventions 8 . This evolution emphasizes the strategic advantage of utilizing MSC-EVs over<br />
direct MSC therapies, as they represent an innovative method for harnessing the full<br />
regenerative potential of mesenchymal stem cells, setting a new st<strong>and</strong>ard in medical treatments.<br />
Culture <strong>and</strong> Expansion of <strong>MSCs</strong> for Extracellular Vesicle<br />
<strong>Production</strong><br />
The origin, culture, <strong>and</strong> expansion of <strong>MSCs</strong> are crucial for EVs production. The choice of<br />
expansion method significantly impacts EVs yield <strong>and</strong> quality.<br />
The field of MSC research faces challenges due to the inherent tendency of primary <strong>MSCs</strong> to<br />
undergo senescence during culture expansion. This limitation has prompted researchers to<br />
explore the generation <strong>and</strong> characterization of immortalized MSC (iMSC) lines as a potential<br />
solution. IMSC lines, such as those created by inducing the expression of human telomerase<br />
reverse transcriptase (hTERT), have been investigated for their ability to offer a reliable <strong>and</strong><br />
scalable source of <strong>MSCs</strong> for EVs production 17 . Studies have indicated that iMSC lines could<br />
serve as a consistent resource for EVs production, which is crucial for various therapeutic<br />
applications. However, it is important to note that i<strong>MSCs</strong> may exhibit functional alterations<br />
compared to primary <strong>MSCs</strong> 18 .<br />
Tel: +49 (0) 89 517 286 59 0<br />
Mail: info@pelobiotech.com
www.pelobiotech.com<br />
This difference in functionality raises concerns about the suitability of i<strong>MSCs</strong> for certain<br />
applications <strong>and</strong> underscores the importance of further research to underst<strong>and</strong> the implications<br />
of iMSC behaviour <strong>and</strong> characteristics. Moreover, the source of <strong>MSCs</strong>, whether primary,<br />
induced pluripotent stem cell (iPSC)-derived, or immortalized, can influence EVs production.<br />
While iPSC derived <strong>MSCs</strong> have shown promise for specific applications, primary <strong>MSCs</strong> are<br />
still preferred in many cases due to their superior supportive capabilities in co-culture<br />
systems 19 . The choice of MSC source is a critical consideration in EVs production, as it can<br />
impact the quantity <strong>and</strong> quality of EVs generated for therapeutic purposes.<br />
The culture media used for MSC expansion can also influence EVs production <strong>and</strong><br />
functionality. The use of defined media has been suggested as advantageous for maintaining<br />
the desired characteristics of <strong>MSCs</strong> <strong>and</strong> their derived EVs 20 . Additionally, it may be necessary<br />
to add special lipid cocktails for high EVs production. The balance between high<br />
proliferation/expansion rates <strong>and</strong> EVs production is a critical consideration. While high<br />
proliferation rates are desirable for obtaining large quantities of <strong>MSCs</strong>, it may lead to<br />
competition for resources, such as lipids, which are essential for both proliferation <strong>and</strong> EVs<br />
biogenesis 21 . Studies have indicated that the efficiency of EVs production may inversely<br />
correlate with the developmental maturity of the MSC donor, further highlighting the<br />
importance of donor selection for optimal EVs yield 21 .<br />
Moreover, the choice of having serum in the cell culture, such as FBS, hPL, or AB serum, can<br />
introduce both variability in proliferation, <strong>and</strong> expansion. Utilizing defined media can help to<br />
overcome these batch-to-batch variabilities <strong>and</strong> ensure consistent functional EVs production 20 .<br />
In conclusion, the culture <strong>and</strong> expansion of <strong>MSCs</strong> for EVs production involve various factors<br />
that influence the quantity <strong>and</strong> quality of EVs. Careful consideration of expansion methods,<br />
culture media, cell source, proliferation rates, <strong>and</strong> serum choice is essential to optimize EVs<br />
production for therapeutic applications. Special lipid cocktails may be necessary to enhance<br />
EVs production efficiency <strong>and</strong> yield, further emphasizing the importance of optimizing culture<br />
conditions for successful EVs-based therapies.<br />
Isolation Techniques for MSC-Derived EVs<br />
Isolating EVs from <strong>MSCs</strong> is a noteworthy area of research, essential for obtaining pure EVs<br />
samples for applications ranging from therapeutic use, drug delivery, regenerative medicine,<br />
<strong>and</strong> tissue engineering. Various methods such as ultracentrifugation, differential<br />
ultracentrifugation, <strong>and</strong> tangential flow filtration are employed, each with its distinct<br />
advantages <strong>and</strong> limitations 4 .<br />
• Ultracentrifugation: Ultracentrifugation is one of the most traditional <strong>and</strong> widely used<br />
methods for Extracellular Vesicles isolation. This technique relies on the application of<br />
extremely high centrifugal forces, typically ranging from 100,000 to 200,000g to<br />
sediment EVs from MSC culture media or other biological fluids. The process involves<br />
multiple centrifugation steps at varying speeds <strong>and</strong> durations to progressively remove<br />
cells, cell debris, <strong>and</strong> larger vesicles, culminating in the sedimentation of EVs.<br />
o<br />
Advantages:<br />
Widely available: The equipment required for ultracentrifugation is available in<br />
most research laboratories.<br />
Tel: +49 (0) 89 517 286 59 0<br />
Mail: info@pelobiotech.com
www.pelobiotech.com<br />
Scalable: It can be adapted for large-volume preparations, making it suitable for<br />
both research <strong>and</strong> clinical applications.<br />
o<br />
Limitations:<br />
Time-consuming: The process is labor-intensive <strong>and</strong> requires several hours to<br />
complete.<br />
Potential for contamination: Co-isolation of protein aggregates or other vesicles<br />
of similar density can occur.<br />
Sample integrity: The high forces applied can potentially damage the EVs or<br />
alter their functional properties.<br />
• Differential Ultracentrifugation<br />
Differential ultracentrifugation refines the basic ultracentrifugation process by<br />
employing a series of centrifugation steps at gradually increasing speeds. This method<br />
allows for more precise separation of EVs from other components based on their size<br />
<strong>and</strong> density.<br />
o<br />
o<br />
Advantages:<br />
Improved purity: By carefully adjusting the centrifugation parameters, it is<br />
possible to enhance the purity of the isolated EVs.<br />
Versatility: It can be used in conjunction with other purification steps to further<br />
increase the yield <strong>and</strong> purity of EVs.<br />
Limitations:<br />
Complexity: The process requires meticulous optimization of centrifugation<br />
speeds <strong>and</strong> times for each specific sample type.<br />
Sample loss: Each centrifugation step may lead to a loss of EVs yield.<br />
• Tangential Flow Filtration (TFF) 32<br />
Tangential flow filtration is a more modern approach that utilizes a crossflow<br />
mechanism, where the sample fluid flows tangentially across the surface of a membrane<br />
filter. This method effectively separates EVs based on their size, allowing them to pass<br />
through the membrane while larger particles are retained.<br />
o<br />
o<br />
Advantages:<br />
Efficiency: TFF can process large volumes of samples in a relatively short<br />
amount of time.<br />
Gentle on samples: The technique is less likely to damage EVs compared to<br />
ultracentrifugation.<br />
Scalability <strong>and</strong> reproducibility: TFF is easily scalable <strong>and</strong> offers high<br />
reproducibility, making it suitable for clinical applications.<br />
Limitations:<br />
Equipment cost: The initial investment for TFF systems can be high.<br />
Membrane maintenance: Over time, the membrane may become clogged with<br />
particles, requiring regular maintenance or replacement.<br />
The choice of an EVs isolation technique depends on various factors, including the source of<br />
<strong>MSCs</strong>, the volume of the sample, the desired purity <strong>and</strong> yield of EVs, <strong>and</strong> the available<br />
Tel: +49 (0) 89 517 286 59 0<br />
Mail: info@pelobiotech.com
www.pelobiotech.com<br />
resources. Each method has its trade-offs; thus, a combination of techniques should be used to<br />
achieve the best results. Continuous advancements in EVs isolation technologies are expected<br />
to enhance the efficiency, yield, <strong>and</strong> purity of EVs preparations.<br />
Enhancing EVs <strong>Production</strong> from <strong>MSCs</strong><br />
Optimizing the production of EVs from <strong>MSCs</strong> can significantly lead to more effective application<br />
possibilities by ensuring that enough potent, high-quality EVs are available for research <strong>and</strong> clinical<br />
therapy.<br />
Currently, several strategies are being developed to boost EVs production:<br />
• Culturing with Bioactive Glass (BG) Ion Products: Culturing <strong>MSCs</strong> with BG ion productenriched<br />
medium significantly increases Extracellular Vesicles production without altering<br />
their inherent characteristics 5 .<br />
• Use of Small Molecules: Identified specific small molecules capable of enhancing<br />
Extracellular Vesicles production in <strong>MSCs</strong>, with ongoing research exploring their effects on<br />
the EVs composition <strong>and</strong> regenerative capacity 6 .<br />
• Preconditioning <strong>and</strong> Engineering: Innovative strategies such as preconditioning <strong>MSCs</strong> <strong>and</strong><br />
engineering EVs are being investigated to amplify the therapeutic activity of MSC-EVs 7 .<br />
Navigating the Evolving L<strong>and</strong>scape of Engineered EVs Therapies:<br />
Opportunity <strong>and</strong> Challenges in Clinical Translation<br />
The l<strong>and</strong>scape of EV-based therapies growing exponentially, with over 150 clinical trials,<br />
spanning various domains such as respiratory disorders, infectious diseases, <strong>and</strong> oncology 9 .<br />
Notably, MSC-EVs are particularly promising, offering a compelling alternative to traditional<br />
stem cell therapies. As we have talked earlier, MSC-EVs can replicate the therapeutic impacts<br />
of their source <strong>MSCs</strong>, with added benefits like reduced size, increased stability, <strong>and</strong> more<br />
versatile administration routes 10 . Various companies are at the forefront of advancing the<br />
therapeutic potential of EVs through the engineering of EVs membrane proteins. These<br />
developments have led to innovative treatments, such as the creation of inhalable COVID-19<br />
vaccines utilizing EVs derived from lung stem cells. The contributions from multiple<br />
companies have played a significant role in driving progress in this field. The exciting world<br />
of engineered Extracellular Vesicles therapy is on the brink of transforming how we approach<br />
healing, opening a whole new world of medical possibilities 11 . Despite the promise of MSC-<br />
EVs, challenges persist in translating these therapies from bench to bedside. Issues concerning<br />
safety, st<strong>and</strong>ardized isolation protocols, <strong>and</strong> EVs characterization require resolution 12 .<br />
Additionally, the heterogeneity of EVs populations, influenced by extracellular environmental<br />
factors, complicates their therapeutic application 13 . A deeper underst<strong>and</strong>ing of exosomal cargo<br />
<strong>and</strong> its disease-specific roles is imperative for the full realization of exosomal potential in<br />
clinical settings.<br />
Lastly, the scale-up of MSC-EVs production for clinical applications encounters significant<br />
difficulties, primarily due to the substantial volume required to treat a single patient. Traditional<br />
volume reduction methods, such as ultracentrifugation, are notably inefficient for this scale,<br />
Tel: +49 (0) 89 517 286 59 0<br />
Mail: info@pelobiotech.com
www.pelobiotech.com<br />
with the maximum processing volume per run capped at under 500 mL, starkly inadequate for<br />
the quantities needed for EV-based therapeutics. This limitation highlights a critical bottleneck<br />
in the transition from laboratory-scale research to clinical applications. Key challenges include<br />
maintaining the purity <strong>and</strong> functionality of EVs, ensuring consistent quality across batches,<br />
source of EVs, isolation methods, <strong>and</strong> biodistribution, which are crucial for the successful<br />
translation of MSC-EVs into clinical use.<br />
In summary, the synergy between <strong>MSCs</strong> <strong>and</strong> EVs is illuminating new frontiers in regenerative<br />
medicine. As we unravel the complexities of MSC-EVs, we edge closer to a new epoch of<br />
therapeutic interventions that are safer, more efficacious, <strong>and</strong> transformative. These diminutive<br />
vesicles, emerging from the intricacies of cellular communication, hold the potential to redefine<br />
medical treatments, offering renewed hope for patients worldwide.<br />
References<br />
1. Cuesta-Gomez, N., Graham, G. J., & Campbell, J. (2021). Chemokines <strong>and</strong> their receptors: predictors of the<br />
therapeutic potential of mesenchymal stromal cells. Journal of Translational Medicine, 19(1).<br />
https://doi.org/10.1186/s12967-021-02822-5<br />
2. Ti, D., Hao, H., Tong, C., Liu, J., Dong, L., Zheng, J., … & Han, W. (2015). Lps-preconditioned mesenchymal<br />
stromal cells modify macrophage polarization for resolution of chronic inflammation via Extracellular Vesiclesshuttled<br />
let-7b. Journal of Translational Medicine, 13(1). https://doi.org/10.1186/s12967-015-0642-6<br />
3. Tian, J., Popal, M. S., Zhao, Y., Liu, Y., Chen, K., & Liu, Y. (2019). Interplay between Extracellular Vesicless <strong>and</strong><br />
autophagy in cardiovascular diseases: novel promising target for diagnostic <strong>and</strong> therapeutic application. Aging <strong>and</strong><br />
Disease, 10(6), 1302. https://doi.org/10.14336/ad.2018.1020<br />
4. Helwa I, Cai J, Drewry MD, Zimmerman A, Dinkins MB, Khaled ML, Seremwe M, Dismuke WM, Bieberich E,<br />
Stamer WD, Hamrick MW, Liu Y. A Comparative Study of Serum Extracellular Vesicles Isolation Using<br />
Differential Ultracentrifugation <strong>and</strong> Three Commercial Reagents. PLoS One. 2017 Jan 23;12(1): e0170628. doi:<br />
10.1371/journal.pone.0170628.<br />
5. Zhi Wu, Dan He, Haiyan Li. Bioglass enhances the production of Extracellular Vesicless <strong>and</strong> improves their<br />
capability of promoting vascularization. Bioactive materials (2021) doi: 10.1016/j.bioactmat.2020.09.011.<br />
6. Wang J, Bonacquisti EE, Brown AD, Nguyen J. Boosting the Biogenesis <strong>and</strong> Secretion of Mesenchymal Stem Cell-<br />
Derived Extracellular Vesicless. Cells. 2020 Mar 9;9(3):660. doi: 10.3390/cells9030660.<br />
7. Chen S, Sun F, Qian H, Xu W, Jiang J. Preconditioning <strong>and</strong> Engineering Strategies for Improving the Efficacy of<br />
Mesenchymal Stem Cell-Derived Extracellular Vesicless in Cell-Free Therapy. Stem Cells Int. 2022 May 14;<br />
2022:1779346. doi: 10.1155/2022/1779346.<br />
8. Lee, J., Park, B., Kim, J., Choo, Y. W., Kim, H. Y., Yoon, J., … & Kim, B. S. (2020). Nanovesicles derived from<br />
iron oxide nanoparticles–incorporated mesenchymal stem cells for cardiac repair. Science Advances, 6(18).<br />
https://doi.org/10.1126/sciadv.aaz0952<br />
9. Mendt, M. C., Rezvani, K., & Shpall, E. J. (2019). Mesenchymal stem cell-derived Extracellular Vesicless for<br />
clinical use. Bone Marrow Transplantation, 54(S2), 789-792. https://doi.org/10.1038/s41409-019-0616-z<br />
10. Levy, O., Kuai, R., Siren, E. M. J., Bhere, D., Milton, Y., Nissar, N., … & Karp, J. M. (2020). Shattering barriers<br />
toward clinically meaningful <strong>MSCs</strong> therapies. Science Advances, 6(30). https://doi.org/10.1126/sciadv.aba6884<br />
11. Yin, K., Wang, S., & Zhao, R. C. (2019). Extracellular Vesicless from mesenchymal stem/stromal cells: a new<br />
therapeutic paradigm. Biomarker Research, 7(1). https://doi.org/10.1186/s40364-019-0159-x<br />
12. Lee, B., Kang, I. H., & Yu, K. (2021). Therapeutic features <strong>and</strong> updated clinical trials of mesenchymal stem cell<br />
(<strong>MSCs</strong>)-derived Extracellular Vesicless. Journal of Clinical Medicine, 10(4), 711.<br />
https://doi.org/10.3390/jcm10040711<br />
13. Ahmadi, M. <strong>and</strong> Rezaie, J. (2020). Ageing <strong>and</strong> mesenchymal stem cells derived Extracellular Vesicless: molecular<br />
insight <strong>and</strong> challenges. Cell Biochemistry <strong>and</strong> Function, 39(1), 60-66. https://doi.org/10.1002/cbf.3602<br />
14. Xu, H., Chen, L., Zhou, S., Li, Y., & Xiang, C. (2020). Multifunctional role of micrornas in mesenchymal stem cellderived<br />
Extracellular Vesicless in treatment of diseases. World Journal of Stem Cells, 12(11), 1276-1294.<br />
https://doi.org/10.4252/wjsc.v12.i11.1276<br />
15. Arnold I. Caplan, Diego Correa, The MSC: An Injury Drugstore. Volume 9, Issue 1, 8 July 2011, Pages 11-15.<br />
https://doi.org/10.1016/j.stem.2011.06.008<br />
16. BioRender. 2023. “Sources <strong>and</strong> Potential Applications of Mesenchymal Stromal Cells” https://www.biorender.com/.<br />
17. Burk, J., Holl<strong>and</strong>, H., Lauermann, A., May, T., Siedlaczek, P., Charwat, V., … & Kasper, C. (2019). Generation <strong>and</strong><br />
characterization of a functional human adipose‐derived multipotent mesenchymal stromal cell line. Biotechnology<br />
<strong>and</strong> Bioengineering, 116(6), 1417-1426. https://doi.org/10.1002/bit.26950<br />
Tel: +49 (0) 89 517 286 59 0<br />
Mail: info@pelobiotech.com
www.pelobiotech.com<br />
18. Piñeiro-Ramil, M., Sanjurjo-Rodríguez, C., Rodríguez-Fernández, S., Castro-Viñuelas, R., Hermida-Gómez, T.,<br />
Blanco, F., … & Díaz-Prado, S. (2021). Generation of mesenchymal cell lines derived from aged donors.<br />
International Journal of Molecular Sciences, 22(19), 10667. https://doi.org/10.3390/ijms221910667<br />
19. Vasko, T., Frobel, J., Lubberich, R., Goecke, T., & Wagner, W. (2016). Ipsc-derived mesenchymal stromal cells are<br />
less supportive than primary mscs for co-culture of hematopoietic progenitor cells. Journal of Hematology &<br />
Oncology, 9(1). https://doi.org/10.1186/s13045-016-0273-2<br />
20. Wang, J., Bonacquisti, E., Brown, A., & Nguyen, J. (2020). Boosting the biogenesis <strong>and</strong> secretion of mesenchymal<br />
stem cell-derived Extracellular Vesicless. Cells, 9(3), 660. https://doi.org/10.3390/cells9030660<br />
21. Chen, T., Yeo, R., Arslan, F., Yin, Y., Tan, S., Lai, R., … & Lim, S. (2013). Efficiency of Extracellular Vesicles<br />
production correlates inversely with the developmental maturity of msc donor. Journal of Stem Cell Research &<br />
Therapy, 3(3). https://doi.org/10.4172/2157-7633.1000145<br />
22. Dominici, M., Blanc, K. L., Mueller, I., Slaper‐Cortenbach, I., Marini, F. C., Krause, D. S., … & Horwitz, E. M.<br />
(2006). Minimal criteria for defining multipotent mesenchymal stromal cells. the international society for cellular<br />
therapy position statement. Cytotherapy, 8(4), 315-317. https://doi.org/10.1080/14653240600855905<br />
23. Murray, I. R., Chahla, J., Safran, M. R., Krych, A. J., Saris, D. B., Caplan, A. I., … & Nakamura, N. (2019).<br />
International expert consensus on a cell therapy communication tool: doses. Journal of Bone <strong>and</strong> Joint Surgery,<br />
101(10), 904-911. https://doi.org/10.2106/jbjs.18.00915<br />
24. Caplan AI. Mesenchymal Stem Cells: Time to Change the Name! Stem Cells Transl Med. 2017 Jun;6(6):1445-1451.<br />
doi: 10.1002/sctm.17-0051. Epub 2017 Apr 28. PMID: 28452204; PMCID: PMC5689741.<br />
25. Lindner U, Kramer J, Rohwedel J, Schlenke P. Mesenchymal Stem or Stromal Cells: Toward a Better Underst<strong>and</strong>ing<br />
of Their Biology? Transfus Med Hemother. 2010 Apr;37(2):75-83. doi: 10.1159/000290897. Epub 2010 Mar 15.<br />
PMID: 20737049; PMCID: PMC2914415.<br />
26. Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj,<br />
Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for<br />
Cellular Therapy position statement. Cytotherapy. 2006;8(4):315-7. doi: 10.1080/14653240600855905. PMID:<br />
16923606.<br />
27. Elahi FM, Farwell DG, Nolta JA, Anderson JD. Preclinical translation of Extracellular Vesicless derived from<br />
mesenchymal stem/stromal cells. Stem Cells. 2020 Jan;38(1):15-21. doi: 10.1002/stem.3061. Epub 2019 Oct 1.<br />
PMID: 31381842; PMCID: PMC7004029.<br />
28. Yáñez-Mó M, Silj<strong>and</strong>er PR, Andreu Z, Zavec AB, Borràs FE, Buzas EI, Buzas K, Casal E, Cappello F, Carvalho J,<br />
Colás E, Cordeiro-da Silva A, Fais S, Falcon-Perez JM, Ghobrial IM, Giebel B, Gimona M, Graner M, Gursel I,<br />
Gursel M, Heegaard NH, Hendrix A, Kierulf P, Kokubun K, Kosanovic M, Kralj-Iglic V, Krämer-Albers EM,<br />
Laitinen S, Lässer C, Lener T, Ligeti E, Linē A, Lipps G, Llorente A, Lötvall J, Manček-Keber M, Marcilla A,<br />
Mittelbrunn M, Nazarenko I, Nolte-'t Hoen EN, Nyman TA, O'Driscoll L, Olivan M, Oliveira C, Pállinger É, Del<br />
Portillo HA, Reventós J, Rigau M, Rohde E, Sammar M, Sánchez-Madrid F, Santarém N, Schallmoser K, Ostenfeld<br />
MS, Stoorvogel W, Stukelj R, Van der Grein SG, Vasconcelos MH, Wauben MH, De Wever O. Biological properties<br />
of extracellular vesicles <strong>and</strong> their physiological functions. J Extracell Vesicles. 2015 May 14;4:27066. doi:<br />
10.3402/jev.v4.27066. PMID: 25979354; PMCID: PMC4433489.<br />
29. Lener T, Gimona M, Aigner L, Börger V, Buzas E, Camussi G, Chaput N, Chatterjee D, Court FA, Del Portillo HA,<br />
O'Driscoll L, Fais S, Falcon-Perez JM, Felderhoff-Mueser U, Fraile L, Gho YS, Görgens A, Gupta RC, Hendrix A,<br />
Hermann DM, Hill AF, Hochberg F, Horn PA, de Kleijn D, Kordelas L, Kramer BW, Krämer-Albers EM, Laner-<br />
Plamberger S, Laitinen S, Leonardi T, Lorenowicz MJ, Lim SK, Lötvall J, Maguire CA, Marcilla A, Nazarenko I,<br />
Ochiya T, Patel T, Pedersen S, Pocsfalvi G, Pluchino S, Quesenberry P, Reischl IG, Rivera FJ, Sanzenbacher R,<br />
Schallmoser K, Slaper-Cortenbach I, Strunk D, Tonn T, Vader P, van Balkom BW, Wauben M, Andaloussi SE,<br />
Théry C, Rohde E, Giebel B. Applying extracellular vesicles based therapeutics in clinical trials - an ISEV position<br />
paper. J Extracell Vesicles. 2015 Dec 31;4:30087. doi: 10.3402/jev.v4.30087. PMID: 26725829; PMCID:<br />
PMC4698466.<br />
30. Agnes T. Reiner, Kenneth W. Witwer, Bas W.M. van Balkom, Joel de Beer, Chaya Brodie, R<strong>and</strong>olph L. Corteling,<br />
Susanne Gabrielsson, Mario Gimona, Ahmed G. Ibrahim, Dominique de Kleijn, Charles P. Lai, Jan Lötvall,<br />
Hern<strong>and</strong>o A. del Portillo, Ilona G. Reischl, Milad Riazifar, Carlos Salomon, Hidetoshi Tahara, Wei Seong Toh,<br />
Marca H.M. Wauben, Vicky K. Yang, Yijun Yang, Ronne Wee Yeh Yeo, Hang Yin, Bernd Giebel, Eva Rohde, Sai<br />
Kiang Lim, Concise <strong>Review</strong>: Developing Best-Practice Models for the Therapeutic Use of Extracellular Vesicles,<br />
Stem Cells Translational Medicine, Volume 6, Issue 8, August 2017, Pages 1730–1739,<br />
https://doi.org/10.1002/sctm.17-0055<br />
31. Dirk M. Hermann, Thorsten R. Doeppner, <strong>and</strong> Bernd Giebel, "Extracellular Vesicles as Diagnostic Tool in Transient<br />
Ischemic Attack <strong>and</strong> Ischemic Stroke," Stroke, vol. 52, no. 9, pp. 3348–3350, Aug. 2021.<br />
https://doi.org/10.1161/STROKEAHA.121.036150<br />
32. Börger, V., Dittrich, R., Staubach, S., Zumegen, S., Horn, P., & Giebel, B. (2019). Tangential flow filtration, a<br />
potential method for the scaled preparation of extracellular vesicles. Cytotherapy, 21(5), S89.<br />
https://doi.org/10.1016/j.jcyt.2019.03.431​<br />
33. Zhang K, Chen S, Sun H, Wang L, Li H, Zhao J, Zhang C, Li N, Guo Z, Han Z, Han ZC, Zheng G, Chen X, Li Z.<br />
In vivo two-photon microscopy reveals the contribution of Sox9+ cell to kidney regeneration in a mouse model with<br />
extracellular vesicle treatment. J Biol Chem. 2020 Aug 21;295(34):12203-12213. doi: 10.1074/jbc.RA120.012732.<br />
Epub 2020 Jul 8. PMID: 32641493; PMCID: PMC7443503.<br />
Tel: +49 (0) 89 517 286 59 0<br />
Mail: info@pelobiotech.com