Worldwide Open Proficiency Test: Determination of ... - Nucleus - IAEA
Worldwide Open Proficiency Test: Determination of ... - Nucleus - IAEA
Worldwide Open Proficiency Test: Determination of ... - Nucleus - IAEA
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
program achieving complete sample dissolution. The solutions <strong>of</strong> the residues were combined<br />
with their supernatants, and then evaporated with three portions <strong>of</strong> 5 ml <strong>of</strong> 65% HNO3 to<br />
remove HF and than dissolved in 30 ml <strong>of</strong> 2M HCl and 0.1 g H3BO3.<br />
Radiochemical Separations<br />
After sample digestion, polonium and lead were separated using the method proposed by<br />
Vajda et. al [2]. The solution was loaded on Sr Resin column preconditioned in advance with<br />
100 ml 2 M HCl. The column was rinsed with 100 ml <strong>of</strong> 2 M HCl and 25 ml 6 M HNO3 to<br />
remove the non-retained ions. The effluent and washing solutions were combined and used<br />
for analysis <strong>of</strong> uranium and thorium. Polonium was stripped with 60ml 6 M HNO3, and then<br />
lead was eluted with 60 ml 6 M HCl. Polonium solution was carefully evaporated to dryness.<br />
The residue was taken with 10 ml 0.5 M HCl transferred into a Teflon deposition cell, the pH<br />
<strong>of</strong> the solution was adjusted to 1 using 6 M NaOH. Polonium was auto-deposited onto silver<br />
disc at 90°C for 90 min with stirring the solution, and then Po-210 was determined by isotope<br />
dilution alpha-spectrometry. The Pb fraction was evaporated 3 times with 2 mL <strong>of</strong> 65%<br />
HNO3. The residue was dissolved in 20 ml 1 M HNO3, add 0.400 g oxalic acid to warm<br />
solution and adjust the pH to 3-5 with NH3(aq) to precipitate Pb-oxalate. The Pb-oxalate<br />
precipitate was filtered through a pre-weighed filter paper (∅ 24 mm). The filter was washed<br />
with 3*1 mL water and 2 mL <strong>of</strong> ethanol, dried in oven at 40-50 o C, cooled in a dessicator and<br />
weighted to determine the mass <strong>of</strong> lead-oxalate and the chemical recovery gravimetrically.<br />
The lead-oxalate precipitate was transferred together with the filter into liquid scintillation<br />
vial, dissolved in 1 mL 6 M HNO3 and mixed it with 14 mL ‘INSTA-GEL PLUS’ liquid<br />
scintillation cocktail. Pb-210 was determined by liquid scintillation spectrometry. Figure 5<br />
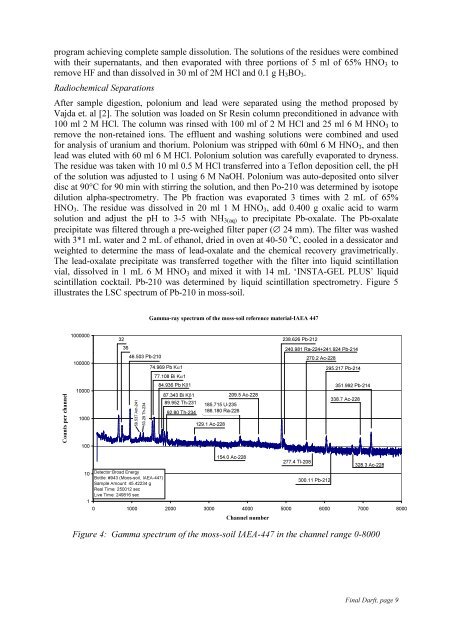
illustrates the LSC spectrum <strong>of</strong> Pb-210 in moss-soil.<br />
Counts per channel<br />
1000000<br />
100000<br />
10000<br />
1000<br />
100<br />
32<br />
36<br />
46.503 Pb-210<br />
59.537 Am-241<br />
63.29 Th-234<br />
Gamma-ray spectrum <strong>of</strong> the moss-soil reference material-<strong>IAEA</strong> 447<br />
74.969 Pb Kα1<br />
77.108 Bi Kα1<br />
84.936 Pb Kβ1<br />
87.343 Bi Kβ1<br />
89.952 Th-231<br />
92.80 Th-234<br />
185.715 U-235<br />
186.180 Ra-226<br />
129.1 Ac-228<br />
209.5 Ac-228<br />
154.0 Ac-228<br />
10 Detector:Broad Energy<br />
Bottle: #943 (Moos-soil, <strong>IAEA</strong>-447)<br />
Sample Amount: 45.42234 g<br />
Real Time: 250012 sec<br />
Live Time: 249816 sec<br />
300.11 Pb-212<br />
1<br />
0 1000 2000 3000 4000 5000 6000 7000 8000<br />
Channel number<br />
238.626 Pb-212<br />
240.981 Ra-224+241.924 Pb-214<br />
277.4 Tl-208<br />
270.2 Ac-228<br />
295.217 Pb-214<br />
351.992 Pb-214<br />
338.7 Ac-228<br />
328.3 Ac-228<br />
Figure 4: Gamma spectrum <strong>of</strong> the moss-soil <strong>IAEA</strong>-447 in the channel range 0-8000<br />
Final Darft, page 9