Superheavy Element Research Superheavy Element Research
Superheavy Element Research Superheavy Element Research
Superheavy Element Research Superheavy Element Research
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
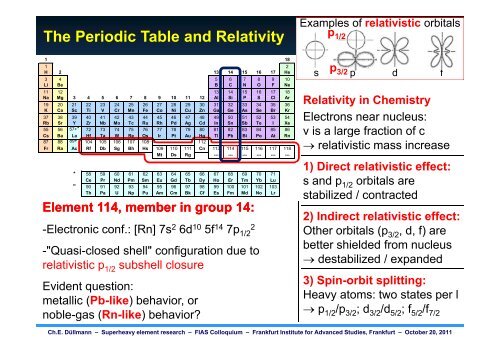
The Periodic Table and Relativity<br />
1 18<br />
1 2<br />
H 2 13 14 15 16 17 He<br />
3 4 5 6 7 8 9 10<br />
Li Be B C N O F Ne<br />
11 12 13 14 15 16 17 18<br />
NNa MMg 3 4 5 6 7 8 9 10 11 12 Al Si P S Cl AAr<br />
19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36<br />
K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr<br />
37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54<br />
Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Xe<br />
55 56 57+* 72 73 74 75 76 77 78 79 80 81 82 83 84 85 86<br />
Cs<br />
87<br />
Ba<br />
88<br />
La<br />
89+"<br />
Hf<br />
104<br />
Ta<br />
105<br />
W<br />
106<br />
Re<br />
107<br />
Os<br />
108<br />
Ir Pt Au Hg<br />
112<br />
Tl Pb Bi Po At Rn<br />
Fr Ra Ac Rf Db Sg Bh Hs 109 110 111 Cn 113 114 115 116 117 118<br />
Mt Ds Rg --- --- --- --- --- ---<br />
* 58 59 60 61 62 63 64 65 66 67 68 69 70 71<br />
Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu<br />
" 90 91 92 93 94 95 96 97 98 99 100 101 102 103<br />
Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No Lr<br />
Rel. effects ~Z2 <strong>Element</strong> 114, � member Pronounced in group in heavy 14: elements<br />
-the -Electronic color of Au: conf.: [Rn] 7s<br />
Au: �E (5d conduct. band � Fermi): 2.4 eV (in vis)<br />
Ag: �E (4d conduct. band � Fermi): 3.5 eV (in UV)<br />
2 6d10 5f14 7p 2<br />
1/2<br />
-"Quasi-closed g ( shell" configuration g ) due ( to )<br />
relativistic p<br />
-the "inert pair" 1/2 subshell closure<br />
effect: stabilized 6s electron pair<br />
reduces Evident valency: question: Tl(I), not Tl(III); Pb(II), not Pb(IV),...<br />
-high metallic t ox.states lli (Pb (Pb-like) lik of light )bbehavior, actinides: h i or expanded 5f orbitals<br />
available noble-gas for (Rn-like) chemical bonding: behavior? U(VI) [but no Nd(VI)]<br />
Examples of relativistic orbitals<br />
p1/2 p 3/2<br />
s p d f<br />
RRelativity l ti it in i Chemistry Ch i t<br />
Electrons near nucleus:<br />
v is a large g fraction of c<br />
� relativistic mass increase<br />
1) Direct relativistic effect:<br />
s and d p1/2 orbitals bit l are<br />
stabilized / contracted<br />
2) Indirect relativistic effect:<br />
Other orbitals (p3/2, d, f) are<br />
better shielded from nucleus<br />
� ddestabilized t bili d / expanded d d<br />
3) Spin-orbit splitting:<br />
Heavy atoms: two states per l<br />
� p1/2/p3/2; d3/2/d5/2; f5/2/f7/2 Ch.E. Düllmann – <strong>Superheavy</strong> element research – FIAS Colloquium – Frankfurt Institute for Advanced Studies, Frankfurt – October 20, 2011