National PMDT Scale-up Plan - India - 2011-12 - TBC India
National PMDT Scale-up Plan - India - 2011-12 - TBC India
National PMDT Scale-up Plan - India - 2011-12 - TBC India
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
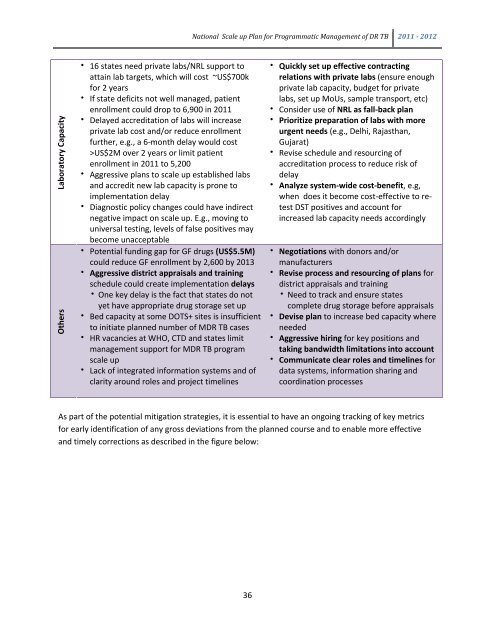
Laboratory Capacity<br />
Others<br />
• 16 states need private labs/NRL s<strong>up</strong>port to<br />
attain lab targets, which will cost ~US$700k<br />
for 2 years<br />
• If state deficits not well managed, patient<br />
enrollment could drop to 6,900 in <strong>2011</strong><br />
• Delayed accreditation of labs will increase<br />
private lab cost and/or reduce enrollment<br />
further, e.g., a 6-month delay would cost<br />
>US$2M over 2 years or limit patient<br />
enrollment in <strong>2011</strong> to 5,200<br />
• Aggressive plans to scale <strong>up</strong> established labs<br />
and accredit new lab capacity is prone to<br />
implementation delay<br />
• Diagnostic policy changes could have indirect<br />
negative impact on scale <strong>up</strong>. E.g., moving to<br />
universal testing, levels of false positives may<br />
become unacceptable<br />
• Potential funding gap for GF drugs (US$5.5M)<br />
could reduce GF enrollment by 2,600 by 2013<br />
• Aggressive district appraisals and training<br />
schedule could create implementation delays<br />
• One key delay is the fact that states do not<br />
yet have appropriate drug storage set <strong>up</strong><br />
• Bed capacity at some DOTS+ sites is insufficient<br />
to initiate planned number of MDR TB cases<br />
• HR vacancies at WHO, CTD and states limit<br />
management s<strong>up</strong>port for MDR TB program<br />
scale <strong>up</strong><br />
• Lack of integrated information systems and of<br />
clarity around roles and project timelines<br />
<strong>National</strong> <strong>Scale</strong> <strong>up</strong> <strong>Plan</strong> for Programmatic Management of DR TB <strong>2011</strong> - 20<strong>12</strong><br />
36<br />
• Quickly set <strong>up</strong> effective contracting<br />
relations with private labs (ensure enough<br />
private lab capacity, budget for private<br />
labs, set <strong>up</strong> MoUs, sample transport, etc)<br />
• Consider use of NRL as fall-back plan<br />
• Prioritize preparation of labs with more<br />
urgent needs (e.g., Delhi, Rajasthan,<br />
Gujarat)<br />
• Revise schedule and resourcing of<br />
accreditation process to reduce risk of<br />
delay<br />
• Analyze system-wide cost-benefit, e.g,<br />
when does it become cost-effective to retest<br />
DST positives and account for<br />
increased lab capacity needs accordingly<br />
• Negotiations with donors and/or<br />
manufacturers<br />
• Revise process and resourcing of plans for<br />
district appraisals and training<br />
• Need to track and ensure states<br />
complete drug storage before appraisals<br />
• Devise plan to increase bed capacity where<br />
needed<br />
• Aggressive hiring for key positions and<br />
taking bandwidth limitations into account<br />
• Communicate clear roles and timelines for<br />
data systems, information sharing and<br />
coordination processes<br />
As part of the potential mitigation strategies, it is essential to have an ongoing tracking of key metrics<br />
for early identification of any gross deviations from the planned course and to enable more effective<br />
and timely corrections as described in the figure below: