- Page 1:
of basic and clinical pharmacology
- Page 4 and 5:
Congress patron 2 HRH Crown Princes
- Page 6 and 7:
Congress President Kim Brøsen Secr
- Page 8 and 9:
General information Registration &
- Page 10 and 11:
Banks, currency & credit cards The
- Page 12 and 13:
Information to presenters ■ The a
- Page 14 and 15:
Hotel Bella Center Copenhagen Groun
- Page 16 and 17:
Programme at a glance Time Hall A2
- Page 18 and 19:
16 General events General events Op
- Page 20 and 21:
Programme outline Plenary lectures
- Page 22 and 23:
FC10 Drugs for half the world: Paed
- Page 24 and 25:
Tue 12:45 W13 Workshop: Novel perip
- Page 26 and 27:
W01 Pharmacology of adrenoceptors:
- Page 28 and 29:
W02 Symposium on advances in GI pha
- Page 30 and 31:
W04 Symposium: Antioxidants as ther
- Page 32 and 33:
Plenary lectures PL01 Plenary lectu
- Page 34 and 35:
FC02 Transmembrane transport: Persp
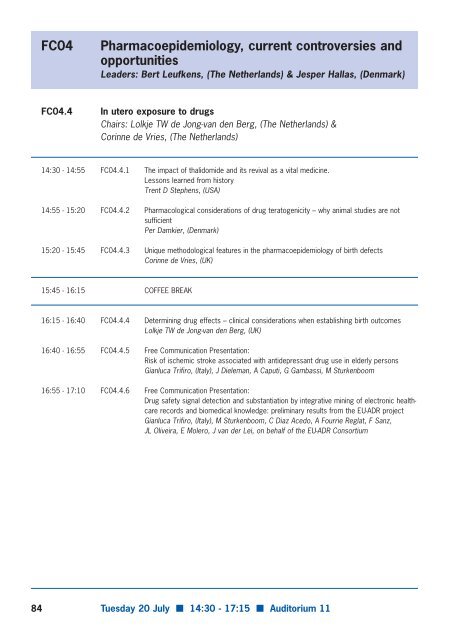
- Page 36 and 37: FC04 Pharmacoepidemiology, current
- Page 38 and 39: FC06 The heart gone wrong: Stabiliz
- Page 40 and 41: FC09 Inflammation and immunopharmac
- Page 42 and 43: W02 Symposium on advances in GI pha
- Page 44 and 45: SS01 Sponsored symposium Sponsored
- Page 46 and 47: W06 Workshop. Effect of disease on
- Page 48 and 49: W08 Workshop. Identifying targets f
- Page 50 and 51: W10 Workshop. Innovative Medicines
- Page 52 and 53: FC01 Clinical pharmacology in the e
- Page 54 and 55: FC03 Ion channels in analgesia and
- Page 56 and 57: FC05 Translational science in the m
- Page 58 and 59: FC06 The heart gone wrong: Stabiliz
- Page 60 and 61: FC08 Development in the treatment o
- Page 62 and 63: Plenary lectures PL04 Plenary lectu
- Page 64 and 65: FC01 Clinical pharmacology in the e
- Page 66 and 67: FC03 Ion channels in analgesia and
- Page 68 and 69: FC05 Translational science in the m
- Page 70 and 71: FC07 Simulation and data modelling
- Page 72 and 73: FC09 Inflammation and immunopharmac
- Page 74 and 75: W12 Workshop. Experimental pain met
- Page 76 and 77: W14 Workshop. Translating the human
- Page 78 and 79: W15 Workshop. The IUPHAR initiative
- Page 80 and 81: W17 Workshop. Standards for PhD Edu
- Page 82 and 83: W19 Workshop. Influence of degenera
- Page 84 and 85: FC02 Transmembrane transport: Persp
- Page 88 and 89: FC06 The heart gone wrong: Stabiliz
- Page 90 and 91: FC08 Development in the treatment o
- Page 92 and 93: PL10 ASPET Plenary Lecture Chair: T
- Page 94 and 95: FC10 Drugs for half the world: Paed
- Page 96 and 97: FC12 Ion channelopathies: New windo
- Page 98 and 99: FC14 Addiction and doping: Neurobio
- Page 100 and 101: FC17 New approaches and targets in
- Page 102 and 103: W04 Symposium: Antioxidants as ther
- Page 104 and 105: W21 Workshop. Inflammation - here,
- Page 106 and 107: W23 Workshop. Applying pharmacogeno
- Page 108 and 109: W25 Workshop. High science in the l
- Page 110 and 111: W27 Workshop. Approaches to medicin
- Page 112 and 113: FC11 G protein-coupled 7TM receptor
- Page 114 and 115: FC13 Maximising benefits and minimi
- Page 116 and 117: FC15 Endothelium in Health and dise
- Page 118 and 119: FC17 New approaches and targets in
- Page 120 and 121: FC18 Nuclear receptor targets for t
- Page 122 and 123: PL16 Plenary Lecture Supported by a
- Page 124 and 125: FC11 G protein-coupled 7TM receptor
- Page 126 and 127: FC13 Maximising benefits and minimi
- Page 128 and 129: FC15 Endothelium in Health and dise
- Page 130 and 131: FC17 New approaches and targets in
- Page 132 and 133: W28 Workshop. The Boehringer-Ingelh
- Page 134 and 135: W30 Workshop. Is eNOS still a valid
- Page 136 and 137:
W32 Workshop. The good, the bad and
- Page 138 and 139:
W34 Workshop. State-of-the-art of b
- Page 140 and 141:
FC10 Drugs for half the world: Pedi
- Page 142 and 143:
FC12 Ion channelopathies: New windo
- Page 144 and 145:
FC14 Addiction and doping: Neurobio
- Page 146 and 147:
FC16 Natural products: Past and fut
- Page 148 and 149:
FC18 Nuclear receptor targets for t
- Page 150 and 151:
PL22 Plenary Lecture Chair: Zhi-Bin
- Page 152 and 153:
FC11 G protein-coupled 7TM receptor
- Page 154 and 155:
FC13 Maximising benefits and minimi
- Page 156 and 157:
FC15 Endothelium in health and dise
- Page 158 and 159:
FC17 New approaches and targets in
- Page 160 and 161:
158
- Page 162 and 163:
FC01 Clinical pharmacology in the e
- Page 164 and 165:
FC01 Clinical pharmacology in the e
- Page 166 and 167:
FC02 Transmembrane transport: Persp
- Page 168 and 169:
FC03 Ion channels in analgesia and
- Page 170 and 171:
W12 Experimental pain methods in de
- Page 172 and 173:
FC04 Pharmacoepidemiology, current
- Page 174 and 175:
FC05 Translational science in the m
- Page 176 and 177:
FC05 Translational science in the m
- Page 178 and 179:
FC06 The heart gone wrong; stabiliz
- Page 180 and 181:
FC07 Simulation and data modelling
- Page 182 and 183:
FC08 Developments in the treatment
- Page 184 and 185:
FC09 Inflammation and immunopharmac
- Page 186 and 187:
FC09 Inflammation and immunopharmac
- Page 188 and 189:
FC09 Inflammation and immunopharmac
- Page 190 and 191:
FC13 Maximising benefits and minimi
- Page 192 and 193:
FC13 Maximising benefits and minimi
- Page 194 and 195:
W03 Targeting TRP channels for pain
- Page 196 and 197:
W19 Influence of degeneration and r
- Page 198 and 199:
W19 Influence of degeneration and r
- Page 200 and 201:
W20 Pharmacology education for a su
- Page 202 and 203:
FC01 Clinical pharmacology in the e
- Page 204 and 205:
FC02 Transmembrane transport: Persp
- Page 206 and 207:
FC02 Transmembrane transport: Persp
- Page 208 and 209:
FC04 Pharmacoepidemiology, current
- Page 210 and 211:
FC05 Translational science in the m
- Page 212 and 213:
FC05 Translational science in the m
- Page 214 and 215:
FC06 The heart gone wrong; stabiliz
- Page 216 and 217:
FC07 Simulation and data modelling
- Page 218 and 219:
FC08 Developments in the treatment
- Page 220 and 221:
FC09 Inflammation and immunopharmac
- Page 222 and 223:
FC09 Inflammation and immunopharmac
- Page 224 and 225:
FC09 Inflammation and immunopharmac
- Page 226 and 227:
FC13 Maximising benefits and minimi
- Page 228 and 229:
FC13 Maximising benefits and minimi
- Page 230 and 231:
FC19 General session Tue 361 Analge
- Page 232 and 233:
FC19 General session - Biologics Tu
- Page 234 and 235:
FC19 General session - Haematology
- Page 236 and 237:
FC19 General session - oncology Tue
- Page 238 and 239:
FC19 General session - oncology Tue
- Page 240 and 241:
FC19 General session - Toxicology T
- Page 242 and 243:
W02 Symposium on advances in GI pha
- Page 244 and 245:
W11 New opportunities in the pharma
- Page 246 and 247:
W23 Applying pharmacogenomics (PGX)
- Page 248 and 249:
FC10 Drugs for half the world: Paed
- Page 250 and 251:
FC11 G protein-coupled 7TM receptor
- Page 252 and 253:
FC11 G protein-coupled 7TM receptor
- Page 254 and 255:
FC12 Ion channelopathies: New windo
- Page 256 and 257:
FC13 Maximising benefits and minimi
- Page 258 and 259:
FC13 Maximising benefits and minimi
- Page 260 and 261:
FC14 Addiction and doping: Neurobio
- Page 262 and 263:
FC15 Endothelium in health and dise
- Page 264 and 265:
FC15 Endothelium in health and dise
- Page 266 and 267:
FC15 Endothelium in health and dise
- Page 268 and 269:
W30 Is eNOS Still a valid therapeut
- Page 270 and 271:
FC16 Natural products: Past and fut
- Page 272 and 273:
FC16 Natural products: Past and fut
- Page 274 and 275:
FC16 Natural products: Past and fut
- Page 276 and 277:
FC17 New approaches and targets in
- Page 278 and 279:
FC17 New approaches and targets in
- Page 280 and 281:
W34 State-of-the-art of basic and c
- Page 282 and 283:
FC10 Drugs for half the world: Paed
- Page 284 and 285:
FC11 G protein-coupled 7TM receptor
- Page 286 and 287:
FC11 G protein-coupled 7TM receptor
- Page 288 and 289:
FC11 G protein-coupled 7TM receptor
- Page 290 and 291:
FC13 Maximising benefits and minimi
- Page 292 and 293:
FC13 Maximising benefits and minimi
- Page 294 and 295:
W29 New horizons in therapeutic dru
- Page 296 and 297:
FC14 Addiction and doping: Neurobio
- Page 298 and 299:
FC15 Endothelium in health and dise
- Page 300 and 301:
FC15 Endothelium in health and dise
- Page 302 and 303:
FC15 Endothelium in health and dise
- Page 304 and 305:
FC16 Natural products: Past and fut
- Page 306 and 307:
FC16 Natural products: Past and fut
- Page 308 and 309:
FC16 Natural products: Past and fut
- Page 310 and 311:
FC16 Natural products: Past and fut
- Page 312 and 313:
FC17 New approaches and targets in
- Page 314 and 315:
FC18 Nuclear receptor targets for t
- Page 316 and 317:
314 Index Name Paper ref. Name Pape
- Page 318 and 319:
316 Index Name Paper ref. Name Pape
- Page 320 and 321:
318 Index Name Paper ref. Name Pape
- Page 322 and 323:
320 Index Name Paper ref. Name Pape
- Page 324 and 325:
322 Index Name Paper ref. Name Pape
- Page 326 and 327:
324 Index Name Paper ref. Name Pape
- Page 328 and 329:
326 Index Name Paper ref. Name Pape
- Page 330 and 331:
328 Index Name Paper ref. Name Pape
- Page 332 and 333:
330 Index Name Paper ref. Name Pape
- Page 334 and 335:
332 Index Name Paper ref. Name Pape
- Page 336 and 337:
334 Index Name Paper ref. Name Pape
- Page 338 and 339:
336 Index Name Paper ref. Name Pape
- Page 340 and 341:
338 Index Name Paper ref. Name Pape
- Page 342 and 343:
340 Index Name Paper ref. Name Pape
- Page 344 and 345:
342 Index Name Paper ref. Name Pape
- Page 346 and 347:
344 Index Name Paper ref. Name Pape
- Page 348 and 349:
346 Index Name Paper ref. Name Pape
- Page 350 and 351:
348 Index Name Paper ref. Name Pape
- Page 352 and 353:
350 Index Name Paper ref. Name Pape
- Page 354 and 355:
352 Index Name Paper ref. Name Pape
- Page 356 and 357:
354 Index Name Paper ref. Name Pape
- Page 358 and 359:
356 Index Name Paper ref. Name Pape
- Page 360 and 361:
358 Index Name Paper ref. Name Pape
- Page 362 and 363:
360 Index Name Paper ref. Name Pape
- Page 364 and 365:
362 Index Name Paper ref. Name Pape
- Page 366 and 367:
364 Index Name Paper ref. Name Pape
- Page 368 and 369:
366 Index Name Paper ref. Name Pape
- Page 370 and 371:
368 Index Name Paper ref. Name Pape
- Page 372 and 373:
370 Index Name Paper ref. Name Pape
- Page 374 and 375:
372 Index Name Paper ref. Name Pape
- Page 376 and 377:
374 Index Name Paper ref. Name Pape
- Page 378 and 379:
376 Index Name Paper ref. Name Pape
- Page 380 and 381:
378 Index Name Paper ref. Name Pape
- Page 382 and 383:
380 Index Name Paper ref. Name Pape
- Page 384 and 385:
382 Notes Notes
- Page 386:
Final Programme printed in Singapor