Chemical & Engineering News Digital Edition - Institute of Materia ...
Chemical & Engineering News Digital Edition - Institute of Materia ...
Chemical & Engineering News Digital Edition - Institute of Materia ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
obsolete, usually around the time companies<br />
lose interest in finding replacements.<br />
The result is a disheartening cycle in which<br />
scientists barely catch up, only to again fall<br />
behind the disease curve.<br />
SMALL COMPANIES are starting to step<br />
in where big pharma left <strong>of</strong>f. Cubist Pharmaceuticals,<br />
where Eisenstein now serves<br />
as senior vice president <strong>of</strong> scientific affairs,<br />
saw promise in daptomycin and licensed it<br />
from Lilly in 1997. As Eisenstein enthusiastically<br />
tells it, in six short years, Cubist scientists<br />
managed to solve the dosing problem—giving<br />
a higher dose less frequently<br />
widened the therapeutic window—and<br />
ushered the drug through late-stage trials,<br />
past regulatory authorities, and to the<br />
market. Last year, Cubist raked in $290<br />
million in sales <strong>of</strong> Cubicin, its brand name<br />
for daptomycin.<br />
Cubist has been the pacesetter for the<br />
cadre <strong>of</strong> biotechs devoted to developing<br />
new antibiotics; today, companies such<br />
as Targanta Therapeutics, Replidyne,<br />
Optimer Pharmaceuticals, and Paratek<br />
Pharmaceuticals have drugs on the edge<br />
<strong>of</strong> commercialization. Like Cubist, many<br />
<strong>of</strong> the tiny biotechs pursuing antibiotics<br />
are built around chemists or physicians<br />
who formerly led anti-infectives R&D and<br />
commercialization efforts at<br />
bigger firms like Lilly, Wyeth,<br />
and Abbott Laboratories that<br />
abandoned the field.<br />
Cubist has proven that a<br />
small company can bring a drug<br />
to market without a partner and<br />
lay the financial groundwork<br />
for a robust new product pipeline.<br />
The company’s Lexington,<br />
Mass., headquarters bustles<br />
with signs <strong>of</strong> expansion, a clear<br />
reminder <strong>of</strong> its recent success.<br />
But the industry needs to<br />
make more progress if antibacterial<br />
drug discovery is to<br />
evolve. Much <strong>of</strong> the late-stage<br />
new product pipeline at biotech<br />
firms consists <strong>of</strong> molecules<br />
licensed from U.S. or Japanese<br />
drug companies. These molecules<br />
are largely derivatives <strong>of</strong><br />
already-marketed compounds<br />
rather than innovative new<br />
classes.<br />
Rather than delve into basic<br />
research, though, biotechs must<br />
focus their limited resources on<br />
their lead compounds—those<br />
well-understood<br />
derivatives <strong>of</strong> older<br />
drugs. That leaves<br />
few research dollars<br />
for the newer<br />
discovery techniques,<br />
such as<br />
high-throughput<br />
screening,<br />
combinatorial<br />
chemistry, and<br />
structure-based<br />
COVER STORY<br />
HO<br />
HO<br />
O<br />
HN<br />
HN<br />
HN<br />
NH<br />
WWW.CEN-ONLINE.ORG 16 APRIL 14, 2008<br />
O<br />
HO<br />
O<br />
O<br />
O<br />
O<br />
H<br />
N<br />
N<br />
H<br />
drug design, needed to<br />
make molecules from scratch.<br />
OH<br />
Cubist’s expanding headquarters<br />
houses its nascent effort to build<br />
the infrastructure for new technologies to<br />
discover antibiotics. “We just had our first<br />
pr<strong>of</strong>itable year,” notes Chet Metcalf, senior<br />
medicinal chemist at Cubist. “Much <strong>of</strong> our<br />
previous R&D work had gone into Cubicin.<br />
We’re just now able to put pr<strong>of</strong>its back into<br />
new molecules.”<br />
Indeed, Lilly’s decision to scrap daptomycin—a<br />
drug that just required a bit more<br />
creative thinking—underscores the particular<br />
challenges <strong>of</strong> discovering and developing<br />
novel antibacterial compounds. Developing<br />
a new antibiotic is a sisyphean task, thanks<br />
to the dauntingly steep evolutionary hill researchers<br />
must climb. “The organisms we’re<br />
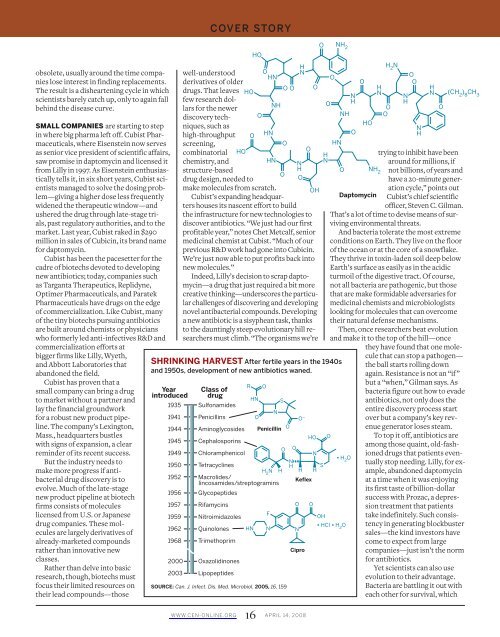
SHRINKING HARVEST After fertile years in the 1940s<br />
and 1950s, development <strong>of</strong> new antibiotics waned.<br />
Year<br />
introduced<br />
Class <strong>of</strong><br />
drug<br />
1935 Sulfonamides<br />
1941 Penicillins<br />
O<br />
O<br />
O<br />
O<br />
H<br />
N<br />
O<br />
NH 2<br />
HN<br />
NH<br />
O<br />
1944 Aminoglycosides Penicillin<br />
1945 Cephalosporins<br />
HO O<br />
1949<br />
1950<br />
1952<br />
O<br />
Chloramphenicol<br />
Tetracyclines<br />
H H<br />
2N Macrolides/<br />
lincosamides/streptogramins<br />
O<br />
N<br />
N<br />
H<br />
S<br />
H H<br />
Keflex<br />
• H2O 1956 Glycopeptides<br />
1957 Rifamycins<br />
O O<br />
1959 Nitroimidazoles<br />
F<br />
OH<br />
1962 Quinolones HN N N<br />
• HCl • H2O 1968 Trimethoprim<br />
2000 Oxazolidinones<br />
2003 Lipopeptides<br />
HN S<br />
SOURCE: Can. J. Infect. Dis. Med. Microbiol. 2005, 16, 159<br />
R<br />
O<br />
O<br />
N<br />
N<br />
H<br />
O<br />
O<br />
O<br />
HO<br />
H<br />
N<br />
NH 2<br />
H 2 N<br />
O<br />
N<br />
H<br />
O<br />
O<br />
N<br />
H<br />
O<br />
O –<br />
Cipro<br />
O<br />
Daptomycin<br />
H<br />
N<br />
O<br />
trying to inhibit have been<br />
around for millions, if<br />
(CH 2 ) 8 CH 3<br />
not billions, <strong>of</strong> years and<br />
have a 20-minute generation<br />
cycle,” points out<br />
Cubist’s chief scientific<br />
<strong>of</strong>ficer, Steven C. Gilman.<br />
That’s a lot <strong>of</strong> time to devise means <strong>of</strong> surviving<br />
environmental threats.<br />
And bacteria tolerate the most extreme<br />
conditions on Earth. They live on the floor<br />
<strong>of</strong> the ocean or at the core <strong>of</strong> a snowflake.<br />
They thrive in toxin-laden soil deep below<br />
Earth’s surface as easily as in the acidic<br />
turmoil <strong>of</strong> the digestive tract. Of course,<br />
not all bacteria are pathogenic, but those<br />
that are make formidable adversaries for<br />
medicinal chemists and microbiologists<br />
looking for molecules that can overcome<br />
their natural defense mechanisms.<br />
Then, once researchers beat evolution<br />
and make it to the top <strong>of</strong> the hill—once<br />
they have found that one mole-<br />
cule that can stop a pathogen—<br />
the ball starts rolling down<br />
again. Resistance is not an “if ”<br />
but a “when,” Gilman says. As<br />
bacteria figure out how to evade<br />
antibiotics, not only does the<br />
entire discovery process start<br />
over but a company’s key revenue<br />
generator loses steam.<br />
To top it <strong>of</strong>f, antibiotics are<br />
among those quaint, old-fashioned<br />
drugs that patients eventually<br />
stop needing. Lilly, for example,<br />
abandoned daptomycin<br />
at a time when it was enjoying<br />
its first taste <strong>of</strong> billion-dollar<br />
success with Prozac, a depression<br />
treatment that patients<br />
take indefinitely. Such consistency<br />
in generating blockbuster<br />
sales—the kind investors have<br />
come to expect from large<br />
companies—just isn’t the norm<br />
for antibiotics.<br />
Yet scientists can also use<br />
evolution to their advantage.<br />
Bacteria are battling it out with<br />
each other for survival, which