Module I Oxidation Reactions - NPTel
Module I Oxidation Reactions - NPTel
Module I Oxidation Reactions - NPTel
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
NPTEL – Chemistry – Reagents and Organic reactions<br />
Me<br />
Mechanism<br />
Me<br />
H<br />
Me<br />
Me<br />
O<br />
O<br />
OMe<br />
SeO 2, reflux<br />
SeO 2, AcOH (cat)<br />
H 2O-dioxane, reflux<br />
Scheme 3<br />
Joint initiative of IITs and IISc – Funded by MHRD Page 38 of 122<br />
O<br />
Me<br />
O<br />
Me<br />
H<br />
Me<br />
O<br />
Me<br />
O<br />
OMe<br />
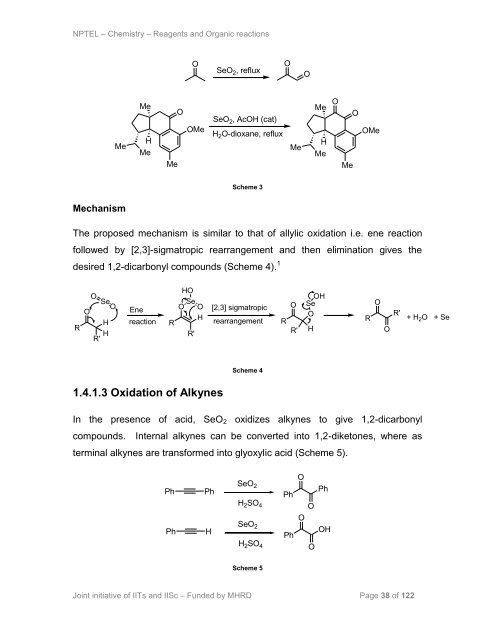
The proposed mechanism is similar to that of allylic oxidation i.e. ene reaction<br />
followed by [2,3]-sigmatropic rearrangement and then elimination gives the<br />
desired 1,2-dicarbonyl compounds (Scheme 4). 1<br />
R<br />
O<br />
O SeO<br />
R'<br />
H<br />
H<br />
Ene<br />
reaction R<br />
HO<br />
Se<br />
O O<br />
H<br />
1.4.1.3 <strong>Oxidation</strong> of Alkynes<br />
R'<br />
[2,3] sigmatropic<br />
rearrangement R<br />
Scheme 4<br />
O<br />
O<br />
R' H<br />
Se OH<br />
In the presence of acid, SeO2 oxidizes alkynes to give 1,2-dicarbonyl<br />
compounds. Internal alkynes can be converted into 1,2-diketones, where as<br />
terminal alkynes are transformed into glyoxylic acid (Scheme 5).<br />
Ph<br />
Ph<br />
Ph<br />
H<br />
SeO 2<br />
H 2SO 4<br />
SeO 2<br />
H 2SO 4<br />
Scheme 5<br />
O<br />
Ph<br />
Ph<br />
O<br />
O<br />
OH<br />
Ph<br />
O<br />
R<br />
O<br />
O<br />
R'<br />
+ H 2O + Se