Low-Valent Titanium Induced Carbonyl Coupling Reactions
Low-Valent Titanium Induced Carbonyl Coupling Reactions
Low-Valent Titanium Induced Carbonyl Coupling Reactions
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
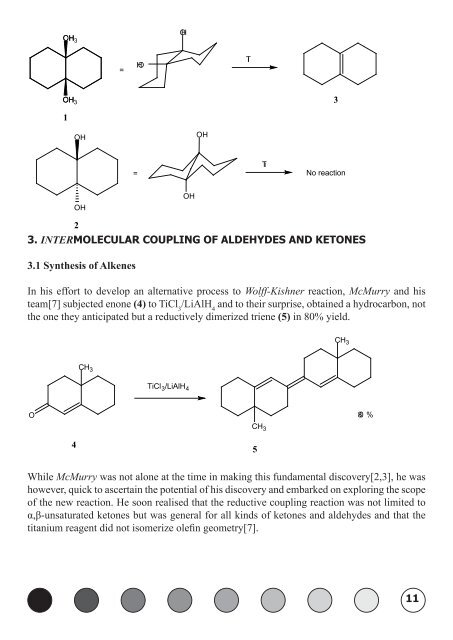
OH CH3 OH CH3 1<br />
OH<br />
OH<br />
2<br />
=<br />
=<br />
HO<br />
OH<br />
OH<br />
OH<br />
Ti<br />
Ti<br />
No reaction<br />
3. InterMOLECULAR COUPLING OF ALdEhydES ANd KETONES<br />
3.1 Synthesis of Alkenes<br />
In his effort to develop an alternative process to Wolff-Kishner reaction, McMurry and his<br />
team[7] subjected enone (4) to TiCl 3 /LiAlH 4 and to their surprise, obtained a hydrocarbon, not<br />
the one they anticipated but a reductively dimerized triene (5) in 80% yield.<br />
O<br />
4<br />
CH 3<br />
TiCl 3/LiAlH 4<br />
While McMurry was not alone at the time in making this fundamental discovery[2,3], he was<br />
however, quick to ascertain the potential of his discovery and embarked on exploring the scope<br />
of the new reaction. He soon realised that the reductive coupling reaction was not limited to<br />
α,β-unsaturated ketones but was general for all kinds of ketones and aldehydes and that the<br />
titanium reagent did not isomerize olefin geometry[7].<br />
CH 3<br />
5<br />
3<br />
CH 3<br />
80 %<br />
11