Antiparkinsonian Agent Piribedil Displays Antagonist Properties at ...
Antiparkinsonian Agent Piribedil Displays Antagonist Properties at ...
Antiparkinsonian Agent Piribedil Displays Antagonist Properties at ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
dependently (and partially) <strong>at</strong>tenu<strong>at</strong>ed the stimul<strong>at</strong>ory action<br />
of NE. The stimul<strong>at</strong>ion elicited by NE, clonidine, and<br />
piribedil was, in each case, abolished by the selective � 2-AR<br />
antagonist <strong>at</strong>ipamezole. Pertussis toxin also abolished the<br />
actions of NE and piribedil.<br />
Inhibition of NE-Activ<strong>at</strong>ed [ 35 S]GTP�S Binding <strong>at</strong><br />
Cerebral � 2-ARs (Figs. 8 and 9). NE (10 �M) elicited a<br />
pronounced increase in [ 35 S]GTP�S binding as quantified in<br />
the insular cortex, amygdala, and l<strong>at</strong>eral septum. This action<br />
of NE was blocked by coincub<strong>at</strong>ion with piribedil (100 �M).<br />
Applied alone, piribedil did not enhance [ 35 S]GTP�S binding.<br />
Indeed, it elicited a mild, although nonsignificant, depression<br />
of basal binding.<br />
<strong>Antagonist</strong> <strong>Properties</strong> <strong>at</strong> h� 1-ARs: Blockade of NE-<br />
Induced [ 3 H]PI Depletion (Fig. 10). In CHO cells stably<br />
expressing h� 1A-ARs, NE elicited a dose-dependent depletion<br />
of membrane-bound [ 3 H]PI, reflecting the positive coupling of<br />
these sites to phospholipase C. In contrast, piribedil did not<br />
modify [ 3 H]PI levels. Indeed, it concentr<strong>at</strong>ion dependently,<br />
albeit weakly, <strong>at</strong>tenu<strong>at</strong>ed the action of NE with a pK b of<br />
5.59 � 0.13.<br />
Activ<strong>at</strong>ion of Locus Ceruleus-Adrenergic Neurons<br />
(Fig. 11). In anesthetized r<strong>at</strong>s, piribedil evoked a dose-dependent<br />
and pronounced increase in the electrical activity of<br />
locus ceruleus-localized, adrenergic cell bodies over a dose<br />
range of 0.125 to 4.0 mg/kg i.v. At its maximally effective dose<br />
(4.0), firing r<strong>at</strong>e was approxim<strong>at</strong>ely doubled rel<strong>at</strong>ive to baseline<br />
values.<br />
Enhancement of Hippocampal Synthesis of NE.<br />
<strong>Piribedil</strong> dose dependently and significantly acceler<strong>at</strong>ed NE<br />
synthesis in the hippocampus, as quantified by determin<strong>at</strong>ion<br />
of its precursor L-dihydroxyphenylalanine in r<strong>at</strong>s pretre<strong>at</strong>ed<br />
with the decarboxylase inhibitor NSD1015. Absolute<br />
levels of L-dihydroxyphenylalanine for vehicle were 0.69 �<br />
0.04 mg/tissue (�100.0 � 5.3%). Expressed rel<strong>at</strong>ive to these<br />
values, the effect of piribedil was as follows: piribedil (2.5<br />
mg/kg s.c.) � 100.2 � 7.1%; piribedil (10.0) � 120.7 � 7.1%;<br />
and piribedil (40.0) � 145.3 � 6.2%; F(3,32) � 9.0, P � 0.001.<br />
Elev<strong>at</strong>ion of Extracellular Levels of NE in Frontal<br />
Cortex and Hippocampus (Fig. 12). <strong>Piribedil</strong> evoked a<br />
dose-dependent (2.5–40.0 mg/kg s.c.) and marked increase in<br />
extracellular levels of NE in the FCX of freely moving r<strong>at</strong>s.<br />
This action was selective inasmuch as levels of 5-HT in the<br />
same samples were not significantly elev<strong>at</strong>ed (d<strong>at</strong>a not<br />
� 2-Adrenoceptors and Parkinson’s Disease 881<br />
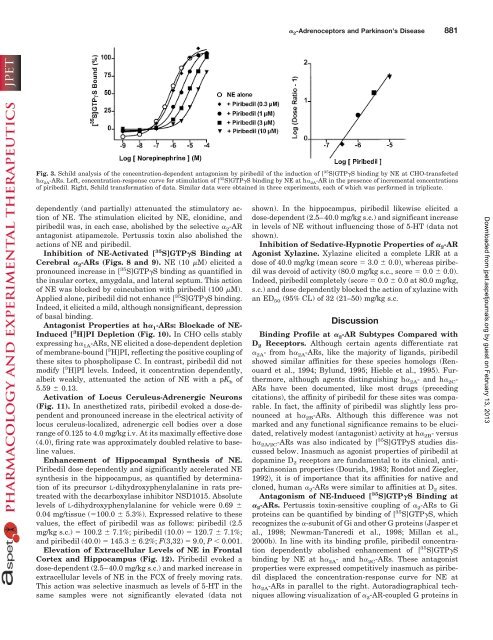
Fig. 3. Schild analysis of the concentr<strong>at</strong>ion-dependent antagonism by piribedil of the induction of [ 35 S]GTP�S binding by NE <strong>at</strong> CHO-transfected<br />
h� 2A-ARs. Left, concentr<strong>at</strong>ion-response curve for stimul<strong>at</strong>ion of [ 35 S]GTP�S binding by NE <strong>at</strong> h� 2A-AR in the presence of incremental concentr<strong>at</strong>ions<br />
of piribedil. Right, Schild transform<strong>at</strong>ion of d<strong>at</strong>a. Similar d<strong>at</strong>a were obtained in three experiments, each of which was performed in triplic<strong>at</strong>e.<br />
shown). In the hippocampus, piribedil likewise elicited a<br />
dose-dependent (2.5–40.0 mg/kg s.c.) and significant increase<br />
in levels of NE without influencing those of 5-HT (d<strong>at</strong>a not<br />
shown).<br />
Inhibition of Sed<strong>at</strong>ive-Hypnotic <strong>Properties</strong> of � 2-AR<br />
Agonist Xylazine. Xylazine elicited a complete LRR <strong>at</strong> a<br />
dose of 40.0 mg/kg (mean score � 3.0 � 0.0), whereas piribedil<br />
was devoid of activity (80.0 mg/kg s.c., score � 0.0 � 0.0).<br />
Indeed, piribedil completely (score � 0.0 � 0.0 <strong>at</strong> 80.0 mg/kg,<br />
s.c.) and dose dependently blocked the action of xylazine with<br />
an ED 50 (95% CL) of 32 (21–50) mg/kg s.c.<br />
Discussion<br />
Binding Profile <strong>at</strong> � 2-AR Subtypes Compared with<br />
D 2 Receptors. Although certain agents differenti<strong>at</strong>e r<strong>at</strong><br />
� 2A- from h� 2A-ARs, like the majority of ligands, piribedil<br />
showed similar affinities for these species homologs (Renouard<br />
et al., 1994; Bylund, 1995; Hieble et al., 1995). Furthermore,<br />
although agents distinguishing h� 2A- and h� 2C-<br />
ARs have been documented, like most drugs (preceding<br />
cit<strong>at</strong>ions), the affinity of piribedil for these sites was comparable.<br />
In fact, the affinity of piribedil was slightly less pronounced<br />
<strong>at</strong> h� 2B-ARs. Although this difference was not<br />
marked and any functional significance remains to be elucid<strong>at</strong>ed,<br />
rel<strong>at</strong>ively modest (antagonist) activity <strong>at</strong> h� 2B- versus<br />
h� 2A/2C-ARs was also indic<strong>at</strong>ed by [ 35 S]GTPyS studies discussed<br />
below. Inasmuch as agonist properties of piribedil <strong>at</strong><br />
dopamine D 2 receptors are fundamental to its clinical, antiparkinsonian<br />
properties (Dourish, 1983; Rondot and Ziegler,<br />
1992), it is of importance th<strong>at</strong> its affinities for n<strong>at</strong>ive and<br />
cloned, human � 2-ARs were similar to affinities <strong>at</strong> D 2 sites.<br />
Antagonism of NE-Induced [ 35 S]GTP�S Binding <strong>at</strong><br />
� 2-ARs. Pertussis toxin-sensitive coupling of � 2-ARs to Gi<br />
proteins can be quantified by binding of [ 35 S]GTP�S, which<br />
recognizes the �-subunit of Gi and other G proteins (Jasper et<br />
al., 1998; Newman-Tancredi et al., 1998; Millan et al.,<br />
2000b). In line with its binding profile, piribedil concentr<strong>at</strong>ion<br />
dependently abolished enhancement of [ 35 S]GTP�S<br />
binding by NE <strong>at</strong> h� 2A- and h� 2C-ARs. These antagonist<br />
properties were expressed competitively inasmuch as piribedil<br />
displaced the concentr<strong>at</strong>ion-response curve for NE <strong>at</strong><br />
h� 2A-ARs in parallel to the right. Autoradiographical techniques<br />
allowing visualiz<strong>at</strong>ion of � 2-AR-coupled G proteins in<br />
Downloaded from<br />
jpet.aspetjournals.org by guest on February 13, 2013