Antiparkinsonian Agent Piribedil Displays Antagonist Properties at ...
Antiparkinsonian Agent Piribedil Displays Antagonist Properties at ...
Antiparkinsonian Agent Piribedil Displays Antagonist Properties at ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
884 Millan et al.<br />
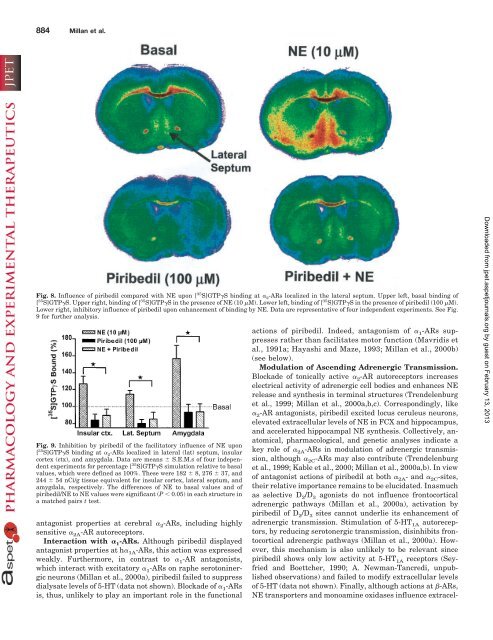
Fig. 8. Influence of piribedil compared with NE upon [ 35 S]GTP�S binding <strong>at</strong> � 2-ARs localized in the l<strong>at</strong>eral septum. Upper left, basal binding of<br />
[ 35 S]GTP�S. Upper right, binding of [ 35 S]GTP�S in the presence of NE (10 �M). Lower left, binding of [ 35 S]GTP�S in the presence of piribedil (100 �M).<br />
Lower right, inhibitory influence of piribedil upon enhancement of binding by NE. D<strong>at</strong>a are represent<strong>at</strong>ive of four independent experiments. See Fig.<br />
9 for further analysis.<br />
Fig. 9. Inhibition by piribedil of the facilit<strong>at</strong>ory influence of NE upon<br />
[ 35 S]GTP�S binding <strong>at</strong> � 2-ARs localized in l<strong>at</strong>eral (l<strong>at</strong>) septum, insular<br />
cortex (ctx), and amygdala. D<strong>at</strong>a are means � S.E.M.s of four independent<br />
experiments for percentage [ 35 S]GTP�S simul<strong>at</strong>ion rel<strong>at</strong>ive to basal<br />
values, which were defined as 100%. These were 182 � 8, 276 � 37, and<br />
244 � 54 nCi/g tissue equivalent for insular cortex, l<strong>at</strong>eral septum, and<br />
amygdala, respectively. The differences of NE to basal values and of<br />
piribedil/NE to NE values were significant (P � 0.05) in each structure in<br />
a m<strong>at</strong>ched pairs t test.<br />
antagonist properties <strong>at</strong> cerebral � 2-ARs, including highly<br />
sensitive � 2A-AR autoreceptors.<br />
Interaction with � 1-ARs. Although piribedil displayed<br />
antagonist properties <strong>at</strong> h� 1A-ARs, this action was expressed<br />
weakly. Furthermore, in contrast to � 1-AR antagonists,<br />
which interact with excit<strong>at</strong>ory � 1-ARs on raphe serotoninergic<br />
neurons (Millan et al., 2000a), piribedil failed to suppress<br />
dialys<strong>at</strong>e levels of 5-HT (d<strong>at</strong>a not shown). Blockade of � 1-ARs<br />
is, thus, unlikely to play an important role in the functional<br />
actions of piribedil. Indeed, antagonism of � 1-ARs suppresses<br />
r<strong>at</strong>her than facilit<strong>at</strong>es motor function (Mavridis et<br />
al., 1991a; Hayashi and Maze, 1993; Millan et al., 2000b)<br />
(see below).<br />
Modul<strong>at</strong>ion of Ascending Adrenergic Transmission.<br />
Blockade of tonically active � 2-AR autoreceptors increases<br />
electrical activity of adrenergic cell bodies and enhances NE<br />
release and synthesis in terminal structures (Trendelenburg<br />
et al., 1999; Millan et al., 2000a,b,c). Correspondingly, like<br />
� 2-AR antagonists, piribedil excited locus ceruleus neurons,<br />
elev<strong>at</strong>ed extracellular levels of NE in FCX and hippocampus,<br />
and acceler<strong>at</strong>ed hippocampal NE synthesis. Collectively, an<strong>at</strong>omical,<br />
pharmacological, and genetic analyses indic<strong>at</strong>e a<br />
key role of � 2A-ARs in modul<strong>at</strong>ion of adrenergic transmission,<br />
although � 2C-ARs may also contribute (Trendelenburg<br />
et al., 1999; Kable et al., 2000; Millan et al., 2000a,b). In view<br />
of antagonist actions of piribedil <strong>at</strong> both � 2A- and � 2C-sites,<br />
their rel<strong>at</strong>ive importance remains to be elucid<strong>at</strong>ed. Inasmuch<br />
as selective D 2/D 3 agonists do not influence frontocortical<br />
adrenergic p<strong>at</strong>hways (Millan et al., 2000a), activ<strong>at</strong>ion by<br />
piribedil of D 2/D 3 sites cannot underlie its enhancement of<br />
adrenergic transmission. Stimul<strong>at</strong>ion of 5-HT 1A autoreceptors,<br />
by reducing serotonergic transmission, disinhibits frontocortical<br />
adrenergic p<strong>at</strong>hways (Millan et al., 2000a). However,<br />
this mechanism is also unlikely to be relevant since<br />
piribedil shows only low activity <strong>at</strong> 5-HT 1A receptors (Seyfried<br />
and Boettcher, 1990; A. Newman-Tancredi, unpublished<br />
observ<strong>at</strong>ions) and failed to modify extracellular levels<br />
of 5-HT (d<strong>at</strong>a not shown). Finally, although actions <strong>at</strong> �-ARs,<br />
NE transporters and monoamine oxidases influence extracel-<br />
Downloaded from<br />
jpet.aspetjournals.org by guest on February 13, 2013