Antiparkinsonian Agent Piribedil Displays Antagonist Properties at ...
Antiparkinsonian Agent Piribedil Displays Antagonist Properties at ...
Antiparkinsonian Agent Piribedil Displays Antagonist Properties at ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
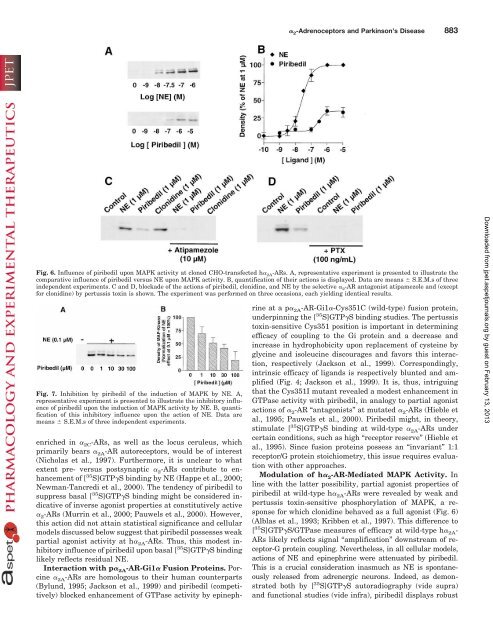
Fig. 6. Influence of piribedil upon MAPK activity <strong>at</strong> cloned CHO-transfected h� 2A-ARs. A, represent<strong>at</strong>ive experiment is presented to illustr<strong>at</strong>e the<br />
compar<strong>at</strong>ive influence of piribedil versus NE upon MAPK activity. B, quantific<strong>at</strong>ion of their actions is displayed. D<strong>at</strong>a are means � S.E.M.s of three<br />
independent experiments. C and D, blockade of the actions of piribedil, clonidine, and NE by the selective � 2-AR antagonist <strong>at</strong>ipamezole and (except<br />
for clonidine) by pertussis toxin is shown. The experiment was performed on three occasions, each yielding identical results.<br />
Fig. 7. Inhibition by piribedil of the induction of MAPK by NE. A,<br />
represent<strong>at</strong>ive experiment is presented to illustr<strong>at</strong>e the inhibitory influence<br />
of piribedil upon the induction of MAPK activity by NE. B, quantific<strong>at</strong>ion<br />
of this inhibitory influence upon the action of NE. D<strong>at</strong>a are<br />
means � S.E.M.s of three independent experiments.<br />
enriched in � 2C-ARs, as well as the locus ceruleus, which<br />
primarily bears � 2A-AR autoreceptors, would be of interest<br />
(Nicholas et al., 1997). Furthermore, it is unclear to wh<strong>at</strong><br />
extent pre- versus postsynaptic � 2-ARs contribute to enhancement<br />
of [ 35 S]GTP�S binding by NE (Happe et al., 2000;<br />
Newman-Tancredi et al., 2000). The tendency of piribedil to<br />
suppress basal [ 35 S]GTP�S binding might be considered indic<strong>at</strong>ive<br />
of inverse agonist properties <strong>at</strong> constitutively active<br />
� 2-ARs (Murrin et al., 2000; Pauwels et al., 2000). However,<br />
this action did not <strong>at</strong>tain st<strong>at</strong>istical significance and cellular<br />
models discussed below suggest th<strong>at</strong> piribedil possesses weak<br />
partial agonist activity <strong>at</strong> h� 2A-ARs. Thus, this modest inhibitory<br />
influence of piribedil upon basal [ 35 S]GTP�S binding<br />
likely reflects residual NE.<br />
Interaction with p� 2A-AR-Gi1� Fusion Proteins. Porcine<br />
� 2A-ARs are homologous to their human counterparts<br />
(Bylund, 1995; Jackson et al., 1999) and piribedil (competitively)<br />
blocked enhancement of GTPase activity by epineph-<br />
� 2-Adrenoceptors and Parkinson’s Disease 883<br />
rine <strong>at</strong> a p� 2A-AR-Gi1�-Cys351C (wild-type) fusion protein,<br />
underpinning the [ 35 S]GTP�S binding studies. The pertussis<br />
toxin-sensitive Cys351 position is important in determining<br />
efficacy of coupling to the Gi protein and a decrease and<br />
increase in hydrophobicity upon replacement of cysteine by<br />
glycine and isoleucine discourages and favors this interaction,<br />
respectively (Jackson et al., 1999). Correspondingly,<br />
intrinsic efficacy of ligands is respectively blunted and amplified<br />
(Fig. 4; Jackson et al., 1999). It is, thus, intriguing<br />
th<strong>at</strong> the Cys351I mutant revealed a modest enhancement in<br />
GTPase activity with piribedil, in analogy to partial agonist<br />
actions of � 2-AR “antagonists” <strong>at</strong> mut<strong>at</strong>ed � 2-ARs (Hieble et<br />
al., 1995; Pauwels et al., 2000). <strong>Piribedil</strong> might, in theory,<br />
stimul<strong>at</strong>e [ 35 S]GTP�S binding <strong>at</strong> wild-type � 2A-ARs under<br />
certain conditions, such as high “receptor reserve” (Hieble et<br />
al., 1995). Since fusion proteins possess an “invariant” 1:1<br />
receptor/G protein stoichiometry, this issue requires evalu<strong>at</strong>ion<br />
with other approaches.<br />
Modul<strong>at</strong>ion of h� 2-AR-Medi<strong>at</strong>ed MAPK Activity. In<br />
line with the l<strong>at</strong>ter possibility, partial agonist properties of<br />
piribedil <strong>at</strong> wild-type h� 2A-ARs were revealed by weak and<br />
pertussis toxin-sensitive phosphoryl<strong>at</strong>ion of MAPK, a response<br />
for which clonidine behaved as a full agonist (Fig. 6)<br />
(Alblas et al., 1993; Kribben et al., 1997). This difference to<br />
[ 35 S]GTP�S/GTPase measures of efficacy <strong>at</strong> wild-type h� 2A-<br />
ARs likely reflects signal “amplific<strong>at</strong>ion” downstream of receptor-G<br />
protein coupling. Nevertheless, in all cellular models,<br />
actions of NE and epinephrine were <strong>at</strong>tenu<strong>at</strong>ed by piribedil.<br />
This is a crucial consider<strong>at</strong>ion inasmuch as NE is spontaneously<br />
released from adrenergic neurons. Indeed, as demonstr<strong>at</strong>ed<br />
both by [ 35 S]GTP�S autoradiography (vide supra)<br />
and functional studies (vide infra), piribedil displays robust<br />
Downloaded from<br />
jpet.aspetjournals.org by guest on February 13, 2013