Nature Immunology
Nature Immunology
Nature Immunology
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
© 2009 <strong>Nature</strong> America, Inc. All rights reserved.<br />
CD11c<br />
CD205<br />
a<br />
CD8<br />
CD103<br />
dDC<br />
CD326<br />
LC<br />
CD8α +<br />
DC<br />
CD103 +<br />
DC<br />
Divided gBT-I/well (×10 3 )<br />
Divided gBT-I/well (×10 3 )<br />
b<br />
c<br />
25<br />
20<br />
15<br />
10<br />
0<br />
DC/well (×10 3 0 1.9 3.8 7.5 15<br />
)<br />
50<br />
40<br />
20<br />
LC<br />
dDC<br />
CD8α + CD103<br />
DC<br />
+ DC<br />
analysis of presentation in the brachial lymph nodes on day 5 showed<br />
presentation by dermal DCs (Fig. 3e) that was not evident on day 4<br />
(Fig. 3c). In this case, presentation by CD8a + DCs was minimal and<br />
presentation by dermal DCs was somewhat less than in the axillary<br />
lymph nodes, possibly because effector CTLs were already actively<br />
killing antigen-presenting cells in this site.<br />
CD103 + DCs present after viral recrudescence<br />
Several groups have reported a third population of skin-derived DCs<br />
that, like Langerhans cells, express langerin 26–28 but, unlike other skinderived<br />
DCs, express CD103 (ref. 26). These cells are restricted mainly<br />
to the dermis and have therefore been called ‘langerin-positive dermal<br />
DCs’, but for simplicity, we will call them ‘CD103 + DCs’ here. These<br />
DCs are resident in the skin and migrate to the lymph nodes after the<br />
skin is painted with tetramethylrhodamine isothiocyanate plus irritant<br />
26 . Both dermal DCs and Langerhans cells migrate after skin is<br />
painted with fluorescein isothiocyanate (FITC) 9 ; here we used painting<br />
with FITC to show that CD103 + DCs migrated in the context of<br />
irritant or infection with HSV-1 (Supplementary Fig. 5 online). These<br />
studies also confirmed the migratory nature of Langerhans cells and<br />
the classical CD103 – dermal DCs and showed a lack of migration by<br />
lymph node–resident CD8a + DCs.<br />
Given the strong presentation by dermal DCs on day 5 reported<br />
above (Fig. 3d), it became imperative to examine the antigenpresenting<br />
activity of contaminating CD103 + DCs in these groups.<br />
To achieve this, we included CD103 staining in our sorting protocol.<br />
Because of limitations in the number of groups that could be sorted,<br />
in subsequent studies we did not include the double-negative DCs<br />
identified above, which represented mainly lymph node–resident<br />
CD8a – DCs that did not seem to contribute to HSV-1-specific<br />
5<br />
30<br />
10<br />
0<br />
Day 2, br LN<br />
Day 5, ax LN<br />
DC/well (×10 3 0 1.9 3.8 7.5 15<br />
)<br />
Figure 5 Many DC subsets present MHC class II–restricted viral antigen to<br />
gDT-II CD4 + T cells. (a,b) Proliferation of 5 10 4 CFSE-labeled HSV-1specific<br />
CD4 + T cells (gDT-II) after 60 h of culture together with serial<br />
dilutions of DC subsets isolated (as described in Fig. 4a) frombrachial<br />
lymph nodes (a) or axillary lymph nodes (b) of mice infected 2 d earlier.<br />
(c,d) Proliferation analysis as described in a,b for DC subsets isolated<br />
from brachial lymph nodes (c) or axillary lymph nodes (d) of mice infected<br />
5 d earlier. Data are pooled from two to four individual experiments<br />
(mean ± s.e.m.).<br />
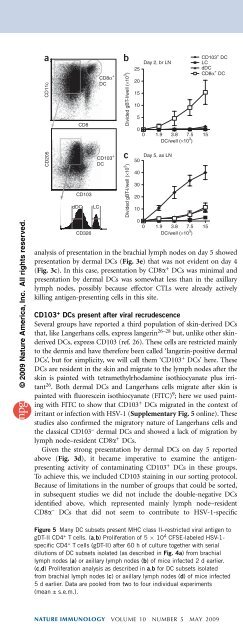
Figure 4 CD103 + DCs present HSV-1 antigens to CD8 + T cells after<br />
secondary viral infection of the skin. (a) Gating strategy for isolation of<br />
CD103 + DCs: after DC enrichment, CD8a + DCs were purified on the<br />
basis of expression of CD11c and CD8a (top; right gate); expression of<br />
CD205 and CD103 (middle) was assessed in CD11c + CD8a – cells (top; left<br />
gate) for the isolation of CD103 + DCs (middle; right gate); and CD103 –<br />
CD11c + CD8a – DCs (middle; left gate) were categorized as Langerhans cells<br />
(CD326 hi ) and dermal DCs (CD326 – ) on the basis of CD326 expression<br />
(bottom; sort gates outlined). Cells of DC subsets purified from lymph<br />
nodes of naive and infected mice are counted in Supplementary Figure 8.<br />
(b,c) Proliferation of 5 10 4 CFSE-labeled gBT-I cells cultured for 60 h<br />
together with serial dilutions of DC subsets (identified as in a) sorted from<br />
brachial lymph nodes 2 d after infection (b) or from axillary lymph nodes<br />
5 d after infection (c). Data are pooled from two to four individual<br />
experiments (mean ± s.e.m.).<br />
CD8 + T cell activation. In this sorting scheme (Fig. 4a), we isolated<br />
CD8a + DCs directly from the CD11c + cells, then isolated CD103 + cells<br />
and separated Langerhans cells and dermal DCs on the basis of CD205<br />
expression and differences in expression of CD326 (Ep-CAM;<br />
expressed by Langerhans cells). We isolated CD8a + DCs first, as<br />
they had low expression of CD103 (Supplementary Fig. 6 online)<br />
that would otherwise potentially result in contamination of the<br />
CD103 + population by this subset.<br />
After sorting the cells as described above, we examined these DC<br />
subsets for their presentation of antigen on day 2 in the brachial<br />
lymph nodes (Fig. 4b) and on day 5 in the axillary lymph nodes<br />
(Fig. 4c), which represent the primary and secondary sites, respectively.<br />
This showed that CD103 + DCs were the dominant migratory<br />
DCs that presented viral antigen on day 5 in the axillary lymph nodes,<br />
whereas little activity by any migratory DC was evident on day 2 in the<br />
brachial lymph nodes. In contrast, CD8a + DCs presented viral antigen<br />
during both phases. Some presentation by dermal DCs and, to a lesser<br />
extent, Langerhans cells was also present on day 5 in the axillary<br />
lymph nodes, but it is difficult to exclude the possibility that this was<br />
not due to contaminating CD103 + DCs, which showed detectable<br />
presentation even with as few as 467 cells (data not shown). These<br />
findings suggest that the CD103 + DCs were dominant among migratory<br />
DCs for the ability to cross-present.<br />
To formally address whether CD103 + DCs that had migrated from<br />
the skin presented viral antigen, we examined presentation on day 5 in<br />
the axillary lymph nodes after labeling the flank skin with FITC at 2 d<br />
Divided gDT-II/well (×10 3 )<br />
Divided gDT-II/well (×10 3 )<br />
a<br />
c<br />
Day 2, br LN<br />
25<br />
20<br />
15<br />
10<br />
5<br />
0<br />
0 1.9 3.8 7.5 15<br />
Day 5, br LN<br />
40<br />
30<br />
20<br />
10<br />
DC/well (×10 3 )<br />
LC<br />
dDC<br />
CD8α + CD103<br />
DC<br />
+ DC<br />
0 1.9 3.8 7.5 15<br />
DC/well (×10 3 )<br />
0<br />
0 1.9 3.8 7.5 15 0 1.9 3.8 7.5 15<br />
DC/well (×10 3 ) DC/well (×10 3 0<br />
)<br />
Divided gDT-II/well (×10 3 )<br />
Divided gDT-II/well (×10 3 )<br />
b<br />
d<br />
25<br />
20<br />
15<br />
10<br />
5<br />
40<br />
30<br />
20<br />
10<br />
Day 2, ax LN<br />
Day 5, ax LN<br />
ARTICLES<br />
NATURE IMMUNOLOGY VOLUME 10 NUMBER 5 MAY 2009 491