Nature Immunology
Nature Immunology
Nature Immunology
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
© 2009 <strong>Nature</strong> America, Inc. All rights reserved.<br />
ARTICLES<br />
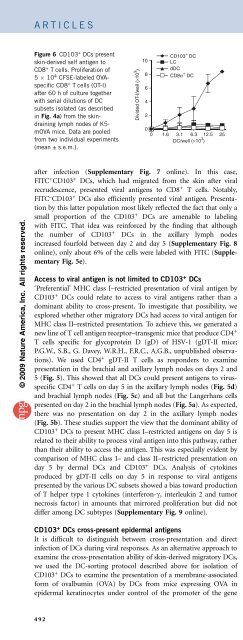
Figure 6 CD103 + DCs present<br />
skin-derived self antigen to<br />
CD8 + T cells. Proliferation of<br />
5 10 4 CFSE-labeled OVAspecific<br />
CD8 + T cells (OT-I)<br />
after 60 h of culture together<br />
with serial dilutions of DC<br />
subsets isolated (as described<br />
in Fig. 4a) from the skindraining<br />
lymph nodes of K5mOVA<br />
mice. Data are pooled<br />
from two individual experiments<br />
(mean ± s.e.m.).<br />
Divided OT-I/well (×10 3 )<br />
LC<br />
dDC<br />
CD8α + CD103<br />
DC<br />
+ DC<br />
after infection (Supplementary Fig. 7 online). In this case,<br />
FITC + CD103 + DCs, which had migrated from the skin after viral<br />
recrudescence, presented viral antigens to CD8 + T cells. Notably,<br />
FITC – CD103 + DCs also efficiently presented viral antigen. Presentation<br />
by this latter population most likely reflected the fact that only a<br />
small proportion of the CD103 + DCs are amenable to labeling<br />
with FITC. That idea was reinforced by the finding that although<br />
the number of CD103 + DCs in the axillary lymph nodes<br />
increased fourfold between day 2 and day 5 (Supplementary Fig. 8<br />
online), only about 6% of the cells were labeled with FITC (Supplementary<br />
Fig. 5e).<br />
Access to viral antigen is not limited to CD103 + DCs<br />
‘Preferential’ MHC class I–restricted presentation of viral antigen by<br />
CD103 + DCs could relate to access to viral antigens rather than a<br />
dominant ability to cross-present. To investigate that possibility, we<br />
explored whether other migratory DCs had access to viral antigen for<br />
MHC class II–restricted presentation. To achieve this, we generated a<br />
new line of T cell antigen receptor–transgenic mice that produce CD4 +<br />
T cells specific for glycoprotein D (gD) of HSV-1 (gDT-II mice;<br />
P.G.W., S.B., G. Davey, W.R.H., F.R.C., A.G.B., unpublished observations).<br />
We used CD4 + gDT-II T cells as responders to examine<br />
presentation in the brachial and axillary lymph nodes on days 2 and<br />
5(Fig. 5). This showed that all DCs could present antigens to virusspecific<br />
CD4 + T cells on day 5 in the axillary lymph nodes (Fig. 5d)<br />
and brachial lymph nodes (Fig. 5c) and all but the Langerhans cells<br />
presented on day 2 in the brachial lymph nodes (Fig. 5a). As expected,<br />
there was no presentation on day 2 in the axillary lymph nodes<br />
(Fig. 5b). These studies support the view that the dominant ability of<br />
CD103 + DCs to present MHC class I–restricted antigens on day 5 is<br />
related to their ability to process viral antigen into this pathway, rather<br />
than their ability to access the antigen. This was especially evident by<br />
comparison of MHC class I– and class II–restricted presentation on<br />
day 5 by dermal DCs and CD103 + DCs. Analysis of cytokines<br />
produced by gDT-II cells on day 5 in response to viral antigens<br />
presented by the various DC subsets showed a bias toward production<br />
of T helper type 1 cytokines (interferon-g, interleukin 2 and tumor<br />
necrosis factor) in amounts that mirrored proliferation but did not<br />
differ among DC subtypes (Supplementary Fig. 9 online).<br />
CD103 + DCs cross-present epidermal antigens<br />
It is difficult to distinguish between cross-presentation and direct<br />
infection of DCs during viral responses. As an alternative approach to<br />
examine the cross-presentation ability of skin-derived migratory DCs,<br />
we used the DC-sorting protocol described above for isolation of<br />
CD103 + DCs to examine the presentation of a membrane-associated<br />
form of ovalbumin (OVA) by DCs from mice expressing OVA in<br />
epidermal keratinocytes under control of the promoter of the gene<br />
10<br />
8<br />
6<br />
4<br />
2<br />
0<br />
DC/well (×10 3 0 1.6 3.1 6.3 12.5 25<br />
)<br />
encoding keratin 5 (K5-mOVA mice). In accordance with our studies<br />
of HSV-1 infection, this showed that CD103 + DCs were the dominant<br />
DC population presenting MHC class I–restricted epidermal skin<br />
antigen in the steady state (Fig. 6). As this antigen was expressed by<br />
keratinocytes but not by DCs 23 , it was apparent that in this case,<br />
CD103 + DCs used cross-presentation.<br />
DISCUSSION<br />
This study has shown that langerin-positive CD103 + dermal DCs are<br />
the dominant migratory DCs of the skin that present MHC class I–<br />
restricted antigens. This dominance most likely relates to their ability<br />
to cross-present, at least for the types of antigens tested here. An<br />
alternative explanation is that presentation of viral antigens by<br />
CD103 + DCs occurs as a consequence of ‘preferential’ infection.<br />
Definitive resolution of this possibility is problematic by most<br />
approaches available, as it is almost impossible to distinguish between<br />
an infected cell and a cell that has captured infected cellular material.<br />
Although some doubt may exist over whether CD103 + DCs use crosspresentation<br />
for HSV-1 antigens, it is likely that this function was<br />
responsible for the presentation of OVA in K5-mOVA mice. The<br />
failure of classical dermal DCs to cross-present OVA in this case might<br />
be explained by limited access to antigen, as OVA is expressed by<br />
epidermal cells. This explanation cannot apply to Langerhans cells,<br />
however, as they reside directly in the region of OVA expression in<br />
K5-mOVA mice 31 . Unfortunately, we have not been able to detect<br />
presentation of OVA on MHC class II by any DC subset in K5-mOVA<br />
mice, which precludes a definitive statement about access by dermal<br />
DCs. However, the ability of all migratory DCs to present viral<br />
antigens on MHC class II during HSV infection of the skin indicates<br />
that all had access to viral antigens, yet only the CD103 + DCs<br />
presented these antigens efficiently on MHC class I.<br />
It is worth considering whether migratory DCs might access viral<br />
antigens in the draining lymph nodes rather than the skin. As mature<br />
DCs are very poor at capturing antigen for presentation 20 , and<br />
migratory DCs have a mature phenotype in the lymph nodes, it is<br />
most likely that viral antigens presented by migratory DCs are<br />
captured in the skin. More importantly, if CD103 + DCs captured<br />
viral antigen in the lymph nodes, then they might be expected to<br />
cross-present it efficiently on both days 2 and 5, as reported for the<br />
CD8a + DCs, yet cross-presentation by CD103 + DCs was absent on day<br />
2. Furthermore, the ability of CD103 + DCs to cross-present skinderived<br />
OVA in the K5-mOVA mice indicated that this is related to<br />
capture in the skin, as capture in the lymph nodes should lead to<br />
similar access and cross-presentation by CD8a + DCs. Although the<br />
possibility that virus was captured in the draining lymph nodes cannot<br />
be completely excluded, the finding that all migratory DCs presented<br />
MHC class II–restricted viral antigens yet only CD103 + DCs presented<br />
these antigens in an MHC class I–restricted way supports the main<br />
point of this report, which is that CD103 + DCs are the dominant<br />
migratory subset that cross-presents viral antigen. Our data are mostly<br />
compatible with published studies of HSV-2 (ref. 32) and HSV-1<br />
(ref. 33) showing that a dermal-like population of DCs provides a<br />
principal antigen-presenting contribution to both CD4 + and CD8 +<br />
T cell antiviral responses. Notably, we specifically separated CD103 +<br />
DCs from classical dermal DCs and identified the CD103 + DCsasthe<br />
key dominant cross-presenting migratory population.<br />
A corollary of the conclusion that CD103 + DCs are the dominant<br />
cross-presenting population of migratory DCs from the skin is that<br />
both Langerhans cells and classical dermal DCs are inefficient at crosspresentation.<br />
Earlier studies have provided evidence that Langerhans<br />
cells do not prime CD8 + T cell responses to HSV 9,16 .Thatdoesnot<br />
492 VOLUME 10 NUMBER 5 MAY 2009 NATURE IMMUNOLOGY