Wassmann und Aulakh - 2000 - The role of rice plants in regulating mechanisms o
Wassmann und Aulakh - 2000 - The role of rice plants in regulating mechanisms o
Wassmann und Aulakh - 2000 - The role of rice plants in regulating mechanisms o
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Biol Fertil Soils (<strong>2000</strong>) 31:20–29 Q Spr<strong>in</strong>ger-Verlag <strong>2000</strong><br />
REVIEW ARTICLE<br />
Re<strong>in</strong>er <strong>Wassmann</strong> 7 Milkha S. <strong>Aulakh</strong><br />
<strong>The</strong> <strong>role</strong> <strong>of</strong> <strong>rice</strong> <strong>plants</strong> <strong>in</strong> regulat<strong>in</strong>g <strong>mechanisms</strong> <strong>of</strong> methane missions<br />
Received: 7 April 1999<br />
Abstract Rice <strong>plants</strong> play a pivotal <strong>role</strong> <strong>in</strong> different<br />
levels <strong>of</strong> the methane (CH 4 ) budget <strong>of</strong> <strong>rice</strong> fields. CH 4<br />
production <strong>in</strong> <strong>rice</strong> fields largely depends on plant-borne<br />
material that can be either decay<strong>in</strong>g tissue or root exudates.<br />
<strong>The</strong> quantity and quality <strong>of</strong> root exudates is affected<br />
by mechanical impedance, presence <strong>of</strong> toxic elements,<br />
nutrient deficiencies, water status <strong>of</strong> grow<strong>in</strong>g<br />
medium, and nitrogenase activity <strong>in</strong> the rhizosphere.<br />
CH 4 oxidation <strong>in</strong> <strong>rice</strong> fields is localized <strong>in</strong> the rhizosphere<br />
where the concentration gradients <strong>of</strong> CH 4 and<br />
oxygen overlap. CH 4 oxidation capacity is a function <strong>of</strong><br />
the downward transport <strong>of</strong> oxygen through the aerenchyma,<br />
which, <strong>in</strong> turn, also acts as a conduit for CH 4<br />
from the soil to the atmosphere. <strong>The</strong> decisive step <strong>in</strong><br />
the passage <strong>of</strong> CH 4 through <strong>rice</strong> plant is the transition<br />
from root to stem. However, <strong>rice</strong> <strong>plants</strong> show an enormous<br />
variety <strong>of</strong> morphological and physiological properties,<br />
<strong>in</strong>clud<strong>in</strong>g differences <strong>in</strong> root exudation and gas<br />
transfer capacity. Comparative studies on different cultivars<br />
are deemed crucial for accomplish<strong>in</strong>g a better <strong>und</strong>erstand<strong>in</strong>g<br />
<strong>of</strong> the <strong>mechanisms</strong> <strong>of</strong> CH 4 consumption <strong>in</strong><br />
the rhizosphere and CH 4 transport through the <strong>rice</strong><br />
plant as well as the <strong>in</strong>teraction <strong>of</strong> these processes. <strong>The</strong><br />
results <strong>of</strong> such studies are considered tools for devis<strong>in</strong>g<br />
mitigation options.<br />
Key words Methane production 7 Methane oxidation 7<br />
Methane emission 7 Rice fields 7 Plant-mediated gas<br />
transfer<br />
R. <strong>Wassmann</strong> (Y) 7 M.S. <strong>Aulakh</strong><br />
International Rice Research Institute, P.O. Box 3127,<br />
1271 Makati City, <strong>The</strong> Philipp<strong>in</strong>es<br />
e-mail: R.<strong>Wassmann</strong>6cgiar.org<br />
Fax: c63-2-891292<br />
R. <strong>Wassmann</strong><br />
Institute for Atmospheric Environmental Research,<br />
Garmisch-Partenkirchen, Germany<br />
M.S. <strong>Aulakh</strong><br />
Department <strong>of</strong> Soils, Punjab Agricultural University, Ludhiana,<br />
Punjab, India<br />
Introduction<br />
<strong>The</strong> atmospheric concentration <strong>of</strong> the radiative active<br />
methane (CH 4 ) gas has <strong>in</strong>creased at rate <strong>of</strong> F0.8% per<br />
year dur<strong>in</strong>g previous decades (IPCC 1994). However,<br />
the concentration <strong>in</strong>crease s<strong>in</strong>ce 1992 has shown large<br />
<strong>in</strong>terannual variation between 0 and 0.5% (Dlugokencky<br />
et al. 1998). Rice cultivation is one <strong>of</strong> the most<br />
important anthropogenic sources <strong>of</strong> atmospheric CH 4<br />
(IPCC 1994). <strong>The</strong> development <strong>of</strong> methods and strategies<br />
to reduce the emission <strong>of</strong> CH 4 from paddy fields is<br />
a central component <strong>of</strong> the ongo<strong>in</strong>g efforts to protect<br />
the earth’s atmosphere and to avert a possible climatic<br />
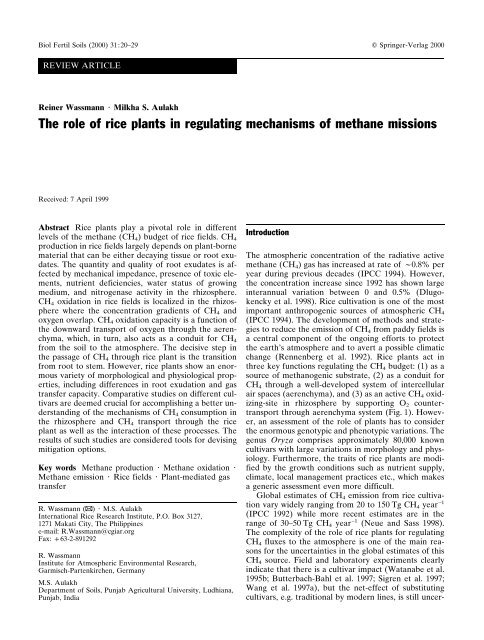
change (Rennenberg et al. 1992). Rice <strong>plants</strong> act <strong>in</strong><br />
three key functions regulat<strong>in</strong>g the CH 4 budget: (1) as a<br />
source <strong>of</strong> methanogenic substrate, (2) as a conduit for<br />
CH 4 through a well-developed system <strong>of</strong> <strong>in</strong>tercellular<br />
air spaces (aerenchyma), and (3) as an active CH 4 oxidiz<strong>in</strong>g-site<br />
<strong>in</strong> rhizosphere by support<strong>in</strong>g O 2 countertransport<br />
through aerenchyma system (Fig. 1). However,<br />
an assessment <strong>of</strong> the <strong>role</strong> <strong>of</strong> <strong>plants</strong> has to consider<br />
the enormous genotypic and phenotypic variations. <strong>The</strong><br />
genus Oryza comprises approximately 80,000 known<br />
cultivars with large variations <strong>in</strong> morphology and physiology.<br />
Furthermore, the traits <strong>of</strong> <strong>rice</strong> <strong>plants</strong> are modified<br />
by the growth conditions such as nutrient supply,<br />
climate, local management practices etc., which makes<br />
a generic assessment even more difficult.<br />
Global estimates <strong>of</strong> CH 4 emission from <strong>rice</strong> cultivation<br />
vary widely rang<strong>in</strong>g from 20 to 150 Tg CH 4 year –1<br />
(IPCC 1992) while more recent estimates are <strong>in</strong> the<br />
range <strong>of</strong> 30–50 Tg CH 4 year –1 (Neue and Sass 1998).<br />
<strong>The</strong> complexity <strong>of</strong> the <strong>role</strong> <strong>of</strong> <strong>rice</strong> <strong>plants</strong> for regulat<strong>in</strong>g<br />
CH 4 fluxes to the atmosphere is one <strong>of</strong> the ma<strong>in</strong> reasons<br />
for the uncerta<strong>in</strong>ties <strong>in</strong> the global estimates <strong>of</strong> this<br />
CH 4 source. Field and laboratory experiments clearly<br />
<strong>in</strong>dicate that there is a cultivar impact (Watanabe et al.<br />
1995b; Butterbach-Bahl et al. 1997; Sigren et al. 1997;<br />
Wang et al. 1997a), but the net-effect <strong>of</strong> substitut<strong>in</strong>g<br />
cultivars, e.g. traditional by modern l<strong>in</strong>es, is still uncer-
21<br />
Fig. 1 Schematic view <strong>of</strong> the<br />
methane budget <strong>of</strong> <strong>rice</strong> fields<br />
with emphasis on plant impacts;<br />
<strong>in</strong>sets depict CH 4 diffusion<br />
through aerenchyma <strong>in</strong> a<br />
root, b root-stem transition<br />
zone, and c a stem-leaf section<br />
zone (modified from Nouchi<br />
and Mariko 1993; <strong>Wassmann</strong><br />
et al. 1998)<br />
ta<strong>in</strong>. This is contrasted by the impact <strong>of</strong> different management<br />
practices, such as organic versus m<strong>in</strong>eral fertilizer,<br />
cont<strong>in</strong>uous flood<strong>in</strong>g versus <strong>in</strong>termittent dra<strong>in</strong>age,<br />
that can be predicted with reasonable accuracy (<strong>Wassmann</strong><br />
et al. 1993a, b; Neue et al. 1997). In view <strong>of</strong> the<br />
future <strong>rice</strong> demand for feed<strong>in</strong>g the <strong>in</strong>creas<strong>in</strong>g world<br />
population, the traits <strong>of</strong> high-yield<strong>in</strong>g <strong>rice</strong> varieties will<br />
affect the CH 4 source strength <strong>of</strong> <strong>rice</strong> cultivation. A<br />
thorough <strong>und</strong>erstand<strong>in</strong>g <strong>of</strong> the <strong>mechanisms</strong> <strong>in</strong>volved is<br />
required to direct the endeavours <strong>of</strong> selection – and<br />
possibly breed<strong>in</strong>g – toward high-yield<strong>in</strong>g <strong>rice</strong> <strong>plants</strong><br />
with a limited emission potential. This paper reviews<br />
and compiles the current state <strong>of</strong> knowledge on <strong>mechanisms</strong><br />
and factors that control plant-mediated and<br />
plant-borne CH 4 emissions as well as the significance <strong>of</strong><br />
this process for the overall CH 4 flux from <strong>rice</strong> fields.<br />
Gaps <strong>in</strong> our knowledge and future research needs are<br />
<strong>in</strong>dicated.<br />
CH 4 production <strong>in</strong> soil<br />
Strictly anaerobic conditions and availability <strong>of</strong> readily<br />
decomposable organic substances are essential for the<br />
process <strong>of</strong> CH 4 production <strong>in</strong> soil. Rice <strong>plants</strong> can <strong>in</strong>fluence<br />
the CH 4 production by enhanc<strong>in</strong>g the anaerobiosis<br />
<strong>in</strong> soil as well as by supply<strong>in</strong>g organic compo<strong>und</strong>s<br />
through their root exudates, which have the ability to<br />
readily supply C and energy source to the microorganisms<br />
present <strong>in</strong> the rhizosphere.<br />
Soil redox potential and ecophysiological sequence<br />
<strong>of</strong> reduction processes<br />
Soil anaerobiosis, measured <strong>in</strong> terms <strong>of</strong> redox potential<br />
(Eh), rang<strong>in</strong>g from about –100 to –200 mV, is needed<br />
for the <strong>in</strong>itiation <strong>of</strong> CH 4 production <strong>in</strong> paddy soils (Takai<br />
1961, 1966; Cicerone et al. 1983; Yagi and M<strong>in</strong>ami<br />
1990; L<strong>in</strong>dau et al. 1991; Wang et al. 1993). <strong>The</strong> <strong>in</strong>tensity<br />
and capacity <strong>of</strong> soil reduction are controlled by the<br />
nature and extent <strong>of</strong> organic substances (electron donors),<br />
temperature, degree <strong>of</strong> waterlogg<strong>in</strong>g, and the nature<br />
and quantity <strong>of</strong> electron acceptors (Ponnamperuma<br />
1972). In laboratory experiments (Takai 1961; Watanabe<br />
1984), it has been demonstrated that the ratio <strong>of</strong><br />
CO 2 to CH 4 production <strong>in</strong> several paddy soils varied by<br />
more than a factor <strong>of</strong> 10 and correlated with the relative<br />
ratio <strong>of</strong> total oxidiz<strong>in</strong>g (electron-accept<strong>in</strong>g) capacity<br />
to reduc<strong>in</strong>g (electron-donat<strong>in</strong>g) capacity <strong>of</strong> the soils.<br />
Soils conta<strong>in</strong><strong>in</strong>g high amounts <strong>of</strong> readily decomposable<br />
organic substrates (e.g. acetate, formate, methanol, methylated<br />
am<strong>in</strong>es etc.) and low amounts <strong>of</strong> electron acceptors<br />
(NO 3– , Mn 4c , Fe 3c , SO 4<br />
2–<br />
) are likely to show a<br />
high production <strong>of</strong> CH 4 (Parashar et al. 1991; Yagi et<br />
al. 1994).<br />
As soon as a soil becomes flooded, the trapped O 2 is<br />
respired and <strong>in</strong> the follow<strong>in</strong>g steps, NO 3– , Mn 4c , Fe 3c ,<br />
compo<strong>und</strong>s are reduced successively (Takai and Kamura<br />
1966; Ottow 1969; Yoshida 1975). Dur<strong>in</strong>g these reductive<br />
processes, facultative and obligate anaerobic<br />
bacteria use NO 3– , Mn 4c , Fe 3c , oxides and hydroxides<br />
as alternative electron acceptors to cont<strong>in</strong>ue their energy-conserv<strong>in</strong>g<br />
metabolic reactions at the expense <strong>of</strong><br />
easily decomposable organic material (Takai and Kamura<br />
1966; Munch and Ottow 1977; Ottow and Munch<br />
1978). For example, Munch and Ottow (1983) showed<br />
that NO 3– , Mn 4c and Fe 3c are reduced directly by bacterial<br />
reductases and not through chemical processes.<br />
<strong>The</strong> microbial population may even use non-crystall<strong>in</strong>e<br />
Fe 3c oxides <strong>in</strong> preference to crystall<strong>in</strong>e forms as external<br />
electron acceptors (Munch and Ottow 1980).<br />
Rice <strong>plants</strong> can <strong>in</strong>fluence the soil Eh by consum<strong>in</strong>g<br />
O 2 from the rhizosphere (root respiration) and by enhanc<strong>in</strong>g<br />
the supply <strong>of</strong> electron donors, i.e. readily decomposable<br />
organic substrates through root exudates,<br />
sloughed-<strong>of</strong>f tissues and debris, and microbially reduced<br />
Mn 2c and Fe 2c ions and chelates. <strong>The</strong> variability<br />
<strong>in</strong> content and composition <strong>of</strong> exudates <strong>of</strong> different<br />
<strong>rice</strong> <strong>plants</strong> could <strong>in</strong>fluence soil Eh and CH 4 production
22<br />
differently. For <strong>in</strong>stance, Wang (1995) observed lower<br />
Eh values <strong>in</strong> soil with Dular, a traditional variety from<br />
the Philipp<strong>in</strong>es, than with the modern varieties IR 72<br />
and IR 65538 at head<strong>in</strong>g and ripen<strong>in</strong>g growth stages.<br />
Dular showed highest dry matter production result<strong>in</strong>g<br />
<strong>in</strong> higher root weight and, as a consequence, larger root<br />
exudation <strong>of</strong> organic C.<br />
Root exudates<br />
Root exudates conta<strong>in</strong> both high-molecular-weight substances,<br />
ma<strong>in</strong>ly mucilage and ecto-enzymes, as well as<br />
low-molecular-weight substances consist<strong>in</strong>g <strong>of</strong> organic<br />
acids, phenols and am<strong>in</strong>o acids (Andal et al. 1956;<br />
MacRae and Castro 1966; Trolldenier 1977, 1981;<br />
Marschner 1995). <strong>The</strong> amount <strong>of</strong> knowledge available<br />
on exudation from <strong>rice</strong> <strong>plants</strong> is m<strong>in</strong>ute, so that a discussion<br />
on the factors affect<strong>in</strong>g exudation has to rely on<br />
f<strong>in</strong>d<strong>in</strong>gs obta<strong>in</strong>ed with other crops. Total amounts as<br />
well as the proportion <strong>of</strong> different compo<strong>und</strong>s <strong>in</strong> root<br />
exudates vary considerably due to various endogenous<br />
and exogenous factors such as mechanical impedance,<br />
presence <strong>of</strong> toxic elements, nutrient deficiencies, water<br />
status <strong>of</strong> grow<strong>in</strong>g medium, and nitrogenase activity.<br />
Maize grown <strong>in</strong> a nutrient culture solution exuded a<br />
three times lower amount <strong>of</strong> sugars and vitam<strong>in</strong>s than<br />
exudation by <strong>plants</strong> grown <strong>in</strong> a solid substrate (Schönwitz<br />
and Ziegler 1982). Plants <strong>in</strong>crease their root exudation<br />
to improve their ability to tolerate toxic elements<br />
such as Pb, Cd and Al (Horst et al. 1990). High<br />
exudation restricts the uptake <strong>of</strong> these elements by preferential<br />
b<strong>in</strong>d<strong>in</strong>g and immobilization with mucilage<br />
(Horst et al. 1982). Exudation can also help to cope<br />
with nutrient deficiency through mobilization <strong>of</strong> nutrients<br />
at the root-soil <strong>in</strong>terface, e.g. phosphate desorption<br />
from clay surfaces by the polygalacturonic acid<br />
component <strong>of</strong> mucilage (Nagarajah et al. 1970). Under<br />
dry soil condition, high amounts <strong>of</strong> mucilage facilitate<br />
transport <strong>of</strong> nutrients from soil particles to the plasma<br />
membrane <strong>of</strong> root cells (Nambiar 1976).<br />
Plants do not only adjust the quantity but also the<br />
quality <strong>of</strong> root exudates. In general, nutrient deficiencies<br />
are responded to by higher amounts <strong>of</strong> low-molecular-weight<br />
organic acids <strong>in</strong> root exudates (Marschner<br />
1993). If there is a deficiency <strong>of</strong> Mn and Fe, these acids<br />
<strong>in</strong>crease the solubility <strong>of</strong> MnO 2 and Fe-oxides by chelation<br />
facilitat<strong>in</strong>g root uptake (Godo and Reisenauer<br />
1980; Jauregui and Reisenauer 1982). High excretions<br />
<strong>of</strong> citric and malic acid result <strong>in</strong> local acidification <strong>of</strong><br />
the rhizosphere which – <strong>in</strong> turn – mobilizes the spar<strong>in</strong>gly<br />
soluble <strong>in</strong>organic compo<strong>und</strong>s such as phosphates, Fe,<br />
Mn and Zn (H<strong>of</strong>fland et al. 1989; Marschner 1995).<br />
<strong>The</strong>se few examples illustrate that the total amount and<br />
proportion <strong>of</strong> different compo<strong>und</strong>s <strong>in</strong> root exudates<br />
that constitute a dynamic rather than a static plant parameter.<br />
In a study with <strong>rice</strong>, L<strong>in</strong> and You (1989) reported<br />
that root exudates from <strong>rice</strong> conta<strong>in</strong>ed vary<strong>in</strong>g amounts<br />
<strong>of</strong> organic acids, carbohydrates and am<strong>in</strong>o acids.<br />
Among organic acids, citric acid had the highest concentration<br />
followed by malic, succ<strong>in</strong>ic and lactic acid <strong>in</strong><br />
decreas<strong>in</strong>g order. <strong>The</strong>se f<strong>in</strong>d<strong>in</strong>gs illustrate a large variation<br />
<strong>in</strong> composition and content <strong>of</strong> root exudates <strong>of</strong> different<br />
<strong>rice</strong> varieties (Fig. 2). <strong>The</strong>y further observed that<br />
<strong>in</strong>oculation with Alcaligenes faecalis stimulated the excretion<br />
from <strong>rice</strong> roots which was related to the nitrogenase<br />
activity <strong>in</strong> the rhizosphere. Nagarajan and Prasad<br />
(1983) also <strong>in</strong>dicated that significant differences<br />
could exist among <strong>rice</strong> cultivars <strong>in</strong> supply<strong>in</strong>g different<br />
amounts as well as <strong>in</strong> the chemical composition <strong>of</strong> their<br />
exudates. More recently, Wang (1995) compared the<br />
quantities <strong>of</strong> organic C released by roots <strong>of</strong> different<br />
cultivars and fo<strong>und</strong> that the total amount <strong>of</strong> C exuded<br />
was closely related to root dry weight (r 2 p0.919) as<br />
well as to above-gro<strong>und</strong> dry matter production<br />
(r 2 p0.954).<br />
<strong>The</strong>se studies help to expla<strong>in</strong> the seasonal variation<br />
<strong>in</strong> CH 4 emission rates that <strong>of</strong>ten follow plant development.<br />
High emission rates at the panicle differentiation/<br />
flower<strong>in</strong>g stage can clearly be attributed to recent<br />
plant-borne material, either root exudates or decay<strong>in</strong>g<br />
root tissue (Watanabe et al. 1997). In laboratory <strong>in</strong>cubations<br />
<strong>of</strong> soil cores, CH 4 production by soil bacteria<br />
was fo<strong>und</strong> to be highest near the soil surface <strong>in</strong> the <strong>rice</strong><br />
row and decreased with depth and distance from the<br />
plant (Sass et al. 1991b). As the season progressed and<br />
the root system expanded to deeper soil and further<br />
away from the plant base, CH 4 production also <strong>in</strong>creased<br />
<strong>in</strong> these soil regions. Similarly a positive correlation<br />
between <strong>rice</strong> yield and CH 4 emissions <strong>of</strong>ten observed<br />
<strong>und</strong>er field conditions (Neue et al. 1997) could<br />
be due to a close relationship between plant size and C<br />
Fig. 2 Content <strong>of</strong> different organic acids and carbohydrates <strong>in</strong><br />
root exudates <strong>of</strong> four <strong>rice</strong> cultivars from Ch<strong>in</strong>a (drawn from data<br />
<strong>of</strong> L<strong>in</strong> and You 1989)
23<br />
excreted from roots which – <strong>in</strong> turn – <strong>in</strong>fluence CH 4<br />
production.<br />
From these studies, it is obvious that <strong>rice</strong> <strong>plants</strong> play<br />
a key <strong>role</strong> <strong>in</strong> the production <strong>of</strong> CH 4 <strong>in</strong> soils. However,<br />
unfortunately most <strong>of</strong> these studies are based on the<br />
assumption that changes <strong>in</strong> CH 4 efflux rates reflect<br />
changes <strong>in</strong> CH 4 production. This conclusion may not be<br />
valid s<strong>in</strong>ce CH 4 efflux is the net result <strong>of</strong> two opposite<br />
<strong>mechanisms</strong> – production and oxidation <strong>of</strong> CH 4 .<br />
Effects on CH 4 oxidation <strong>in</strong> soil<br />
CH 4 is consumed <strong>in</strong> soil by bacteria that are strictly<br />
obligate aerobes (Papen and Rennenberg 1990). <strong>The</strong>se<br />
bacteria can use CH 4 , but also a few other C compo<strong>und</strong>s<br />
such as methanol as a substrate. As they require<br />
molecular O 2 (Bedard and Knowles 1989; K<strong>in</strong>g<br />
1992; Knowles 1993), methanotrophs occur and are active<br />
<strong>in</strong> oxic-anoxic <strong>in</strong>terfaces where concentration gradients<br />
<strong>of</strong> CH 4 and O 2 overlap. In flooded <strong>rice</strong> fields,<br />
these conditions are generally conf<strong>in</strong>ed to the rhizosphere<br />
and a th<strong>in</strong> layer <strong>of</strong> the topsoil <strong>in</strong>terfac<strong>in</strong>g with the<br />
water. However, depend<strong>in</strong>g on water regime, O 2 -rich<br />
water may <strong>in</strong>trude <strong>in</strong>to the lower soil layers and temporarily<br />
stimulate CH 4 oxidation.<br />
<strong>The</strong> estimates <strong>of</strong> the proportion <strong>of</strong> locally produced<br />
CH 4 that is oxidized <strong>in</strong> the soil ranges from 58% (Sass<br />
et al. 1991a, b) to 80% and higher (Holzapfel-Pschorn<br />
et al. 1985; Conrad and Rothfuss 1991; Inubushi et al.<br />
1992). Low CH 4 concentrations <strong>in</strong> the floodwater <strong>in</strong><br />
contrast to high concentrations <strong>in</strong> the soils (Holzapfel-<br />
Pschorn and Seiler 1986; Nouchi and Mariko 1993) corroborate<br />
the concept <strong>of</strong> a very efficient removal <strong>of</strong> CH 4<br />
dur<strong>in</strong>g the passage through oxidized layers. <strong>The</strong> rate <strong>of</strong><br />
CH 4 oxidation is <strong>of</strong>ten higher <strong>in</strong> <strong>rice</strong>-planted soil than<br />
<strong>in</strong> unplanted soil (Inubushi et al. 1992). Rice <strong>plants</strong> <strong>in</strong>fluence<br />
CH 4 oxidation <strong>in</strong> two ways, i.e. by diffusion <strong>of</strong><br />
atmospheric O 2 via aerenchyma <strong>in</strong>to the rhizosphere,<br />
and by enzymatic oxidation as measured by N flush <strong>in</strong>hibition<br />
technique (Epp and Chanton 1993) and a-<br />
naphthylam<strong>in</strong>e oxidation method (Wang 1995).<br />
Subsequent to flood<strong>in</strong>g, plant roots encounter<br />
anoxic conditions as a result <strong>of</strong> depletion <strong>of</strong> soil O 2 by<br />
microbial and plant root respiration (Gambrell and Patrick<br />
1978). In order to adapt to the flooded anoxic conditions,<br />
most hydrophytes, <strong>in</strong>clud<strong>in</strong>g <strong>rice</strong>, develop an<br />
aerenchyma system <strong>in</strong> both root and stem for ma<strong>in</strong>ta<strong>in</strong><strong>in</strong>g<br />
aerobic respiration (van Raalte 1941; Armstrong<br />
1978, 1979; Keeley 1979; Grosse and Schroeder 1985).<br />
O 2 diffuses through the aerenchyma to the roots (Barber<br />
et al. 1962) while CO 2 and CH 4 move <strong>in</strong> the <strong>in</strong>verse<br />
direction to the atmosphere. In addition to diffusion,<br />
mass flow could also be an important mechanism <strong>of</strong> air<br />
movement to the submerged parts <strong>of</strong> the plant through<br />
the aerenchyma (Rask<strong>in</strong> and Kende 1985). However,<br />
the pore size <strong>of</strong> the aerenchyma is the ma<strong>in</strong> plant parameter<br />
that controls O 2 transport through the plant to<br />
the rhizosphere and <strong>of</strong>ten shows a positive correlation<br />
with O 2 concentration <strong>in</strong> the rhizosphere (Ueckert et<br />
al. 1990).<br />
Several factors have been reported to affect the O 2<br />
release from the <strong>rice</strong> roots. For <strong>in</strong>stance, metabolic <strong>in</strong>hibitors<br />
such as DNP, NaN 3 and KCN could <strong>in</strong>crease<br />
the O 2 release rate (Ando et al. 1983). <strong>The</strong> soil Eh<br />
could also <strong>in</strong>fluence this process (Kludze et al. 1993).<br />
<strong>The</strong> release rates <strong>of</strong> O 2 were negatively correlated with<br />
temperature but were unaffected by light conditions,<br />
photosynthesis and respiration rates (Ando et al. 1983;<br />
Nouchi et al. 1990).<br />
<strong>The</strong> development <strong>of</strong> the aerenchyma is determ<strong>in</strong>ed<br />
by the <strong>in</strong>tensity <strong>of</strong> anaerobiosis. For example, Kludze et<br />
al. (1994) studied the development <strong>of</strong> aerenchyma <strong>in</strong><br />
<strong>plants</strong> at a soil Eh <strong>of</strong> –250B10 mV as compared to<br />
<strong>plants</strong> <strong>und</strong>er well-aerated conditions (515B25 mV). As<br />
a result <strong>of</strong> enlarged aerenchyma, the root porosity was<br />
<strong>in</strong>creased to 41.4% <strong>in</strong> the flooded <strong>plants</strong> as compared<br />
to only 13.3% <strong>in</strong> non-flooded or dra<strong>in</strong>ed <strong>plants</strong>. Increased<br />
porosity enhanced the transport <strong>of</strong> O 2 from the<br />
atmosphere to the roots; O 2 loss from the roots <strong>in</strong>creased<br />
to 4.6 mmol O 2 g –1 day –1 <strong>in</strong> the flooded <strong>plants</strong><br />
as compared to only 1.4 mmol O 2 g –1 day –1 <strong>in</strong> dra<strong>in</strong>ed<br />
<strong>plants</strong>. Frenzel et al. (1992) detected O 2 down to the<br />
depth <strong>of</strong> 40 mm <strong>in</strong> a flooded soil planted with <strong>rice</strong><br />
whereas it was conf<strong>in</strong>ed to a th<strong>in</strong> surface layer (3.5 mm)<br />
<strong>in</strong> an unplanted soil. This result illustrates the transport<br />
<strong>of</strong> O 2 by <strong>rice</strong> <strong>plants</strong> to the flooded anaerobic soil. <strong>The</strong><br />
supply <strong>of</strong> O 2 by the plant to the rhizosphere <strong>of</strong>ten stimulates<br />
high activities <strong>of</strong> CH 4 -oxidiz<strong>in</strong>g bacteria <strong>in</strong> the<br />
vic<strong>in</strong>ity <strong>of</strong> <strong>rice</strong> roots (Watanabe et al. 1997). De Bont et<br />
al. (1978) counted 10 times more CH 4 -oxidiz<strong>in</strong>g bacteria<br />
<strong>in</strong> the rhizosphere <strong>of</strong> <strong>rice</strong> at the tiller<strong>in</strong>g stage than<br />
<strong>in</strong> the bulk <strong>of</strong> the anaerobic soil and one third more<br />
than <strong>in</strong> the oxidized surface soil-water <strong>in</strong>terface.<br />
Different <strong>rice</strong> cultivars can support different rates <strong>of</strong><br />
CH 4 oxidation by develop<strong>in</strong>g variable root porosity and<br />
oxidation powers. Wang et al. (1997a) fo<strong>und</strong> that at the<br />
tiller<strong>in</strong>g stage, the root air spaces were small and did<br />
not vary among three <strong>rice</strong> cultivars. Root air space cont<strong>in</strong>ued<br />
to <strong>in</strong>crease up to the ripen<strong>in</strong>g stage <strong>in</strong> some cultivars<br />
whereas it decreased <strong>in</strong> the commonly grown cultivar<br />
IR 72. In the study <strong>of</strong> Wang et al. (1997a), dry<br />
matter (Fig. 3a) had a direct effect on the CH 4 emission<br />
rate (Fig. 3b) whereas root porosity (Fig. 3c) is not correlated<br />
to the oxidation power <strong>of</strong> the plant (Fig. 3d).<br />
<strong>The</strong> process <strong>of</strong> CH 4 oxidation is generally considered<br />
to be an important <strong>in</strong> situ s<strong>in</strong>k for CH 4 produced<br />
<strong>in</strong> paddy soils (K<strong>in</strong>g 1992). <strong>The</strong> ratio <strong>of</strong> plant-mediated<br />
versus floodwater-mediated oxidation, however, rema<strong>in</strong>s<br />
unclear. Several experiments <strong>in</strong>dicated that up to<br />
40% <strong>of</strong> the potential CH 4 flux could be oxidized <strong>in</strong> the<br />
rhizosphere (Epp and Chanton 1993; Denier van der<br />
Gon and Neue 1996). Indirect assessments suggested<br />
that 50–90% <strong>of</strong> the CH 4 transported to the rhizosphere<br />
<strong>of</strong> the <strong>rice</strong> plant was oxidized (Frenzel et al. 1992).<br />
However, the impact <strong>of</strong> <strong>rice</strong> variety characteristics on<br />
CH 4 oxidation is not well <strong>und</strong>erstood. Varietal differences<br />
may also become important when discuss<strong>in</strong>g CH 4
24<br />
As mentioned earlier, the amount <strong>of</strong> CH 4 emitted from<br />
the <strong>rice</strong> field to the atmosphere is the balance <strong>of</strong> the<br />
two opposite processes, i.e. CH 4 production and oxidation.<br />
CH 4 escapes from the <strong>rice</strong> field to the atmosphere<br />
via three processes, viz. ebullition, diffusion, and transport<br />
through the <strong>rice</strong> plant. <strong>The</strong> impact <strong>of</strong> <strong>plants</strong> on<br />
these processes is discussed below.<br />
Indirect impact <strong>of</strong> <strong>plants</strong> on ebullition and diffusion<br />
Fig. 3 Variability among three <strong>rice</strong> cultivars: IR72 (high-yield<strong>in</strong>g<br />
dwarf), NPT (new plant type IR65598) and Dular (traditional<br />
tall) <strong>in</strong> a dry matter, b CH 4 emission rate, c root oxidation power<br />
and d root porosity at three growth stages. (Redrawn and modified<br />
from data <strong>of</strong> Wang et al. 1997a,c)<br />
oxidation with<strong>in</strong> the aerenchyma. Even though recent<br />
results <strong>in</strong>dicate the presence <strong>of</strong> methanotrophic activity<br />
associated with roots and, to a lesser extent, lower parts<br />
<strong>of</strong> the stem (Watanabe et al. 1997), the significance <strong>of</strong><br />
CH 4 oxidation dur<strong>in</strong>g passage through the <strong>rice</strong> <strong>plants</strong> is<br />
still unknown.<br />
As noted earlier, most <strong>of</strong> the f<strong>in</strong>d<strong>in</strong>gs on CH 4 production<br />
and oxidation are based on <strong>in</strong>direct estimates<br />
from CH 4 efflux rates. This is essentially due to the lack<br />
<strong>of</strong> appropriate methods. Now techniques for <strong>in</strong>hibit<strong>in</strong>g<br />
oxidation and monitor<strong>in</strong>g CH 4 production rates are<br />
available, e.g. acetylene, dimethyl ether, nitrapyr<strong>in</strong>, methyl<br />
fluoride, propylene oxide formation (Oremland<br />
and Culbertson 1992a,b; Epp and Chanton 1993;<br />
Kludze et al. 1993; Oremland and Taylor 1995; Watanabe<br />
et al. 1995a). <strong>The</strong>refore, future studies should be<br />
able to address the effects <strong>of</strong> <strong>rice</strong> plant traits on both<br />
CH 4 production and oxidation directly. <strong>The</strong> measurement<br />
<strong>of</strong> 13 C ab<strong>und</strong>ance and 13 C fractionation could improve<br />
knowledge <strong>of</strong> the function <strong>of</strong> the rhizosphere <strong>in</strong><br />
CH 4 oxidation.<br />
CH 4 emission to the atmosphere<br />
Ebullition and diffusion are purely physical processes<br />
and, thus, are only <strong>in</strong>directly affected by <strong>rice</strong> <strong>plants</strong>. A<br />
well-developed root system acts as a s<strong>in</strong>k and a barrier<br />
for diffusive upward transport <strong>of</strong> CH 4 . Ebullition dom<strong>in</strong>ates<br />
the overall flux only dur<strong>in</strong>g the <strong>in</strong>itial plant<br />
growth stage (<strong>Wassmann</strong> et al. 1996) and upon disturbance<br />
<strong>of</strong> soil due to weed<strong>in</strong>g, harrow<strong>in</strong>g etc. (Denier<br />
van der Gon et al. 1992). Ebullition rates generally follow<br />
a bimodal pattern over one season with high rates<br />
<strong>in</strong> the early and late seasons (<strong>Wassmann</strong> et al. 1996).<br />
<strong>The</strong> decrease <strong>in</strong> ebullition rate <strong>in</strong> mid-season is attributed<br />
to the <strong>in</strong>creas<strong>in</strong>g CH 4 transfer through the aerenchyma,<br />
reduc<strong>in</strong>g the pool size <strong>of</strong> entrapped CH 4 (<strong>Wassmann</strong><br />
et al. 1996). Dur<strong>in</strong>g this period, emerg<strong>in</strong>g gas<br />
bubbles conta<strong>in</strong> less CH 4 and high amounts <strong>of</strong> N 2<br />
(Chidthaisong and Watanabe 1997a, b). Dur<strong>in</strong>g the later<br />
growth stage, however, CH 4 production is very <strong>in</strong>tense<br />
due to the release <strong>of</strong> organic material from the<br />
plant (Chidthaisong and Watanabe 1997a) which results<br />
<strong>in</strong> <strong>in</strong>creases <strong>in</strong> the CH 4 pool and the ebullition<br />
rates observed dur<strong>in</strong>g this period (<strong>Wassmann</strong> et al.<br />
1996).<br />
<strong>The</strong> mechanism <strong>of</strong> CH 4 transport through the <strong>rice</strong><br />
plant<br />
<strong>The</strong> primary function <strong>of</strong> aerenchyma formation <strong>in</strong> hydrophilic<br />
<strong>plants</strong>, <strong>in</strong>clud<strong>in</strong>g <strong>rice</strong>, is the delivery <strong>of</strong> O 2 to<br />
the roots, but several gases are transferred <strong>in</strong> the <strong>in</strong>verse<br />
direction. Aerenchyma transport <strong>of</strong> CO 2 (Higudchi<br />
1982; Higudchi et al. 1984), N 2 and N 2 O (Mosier et<br />
al. 1990; Prade and Trolldenier 1990; <strong>Aulakh</strong> et al.<br />
1992; Bhadrachalam et al. 1992) and CH 4 (Raimbault et<br />
al. 1977; Cicerone and Shetter 1981; Seiler et al. 1984;<br />
Sebacher et al. 1985; Nouchi et al. 1990; Schütz et al.<br />
1991; Nouchi and Mariko 1993) by <strong>rice</strong> and other <strong>plants</strong><br />
is well documented.<br />
<strong>The</strong> phenomenon <strong>of</strong> CH 4 transport through the <strong>rice</strong><br />
plant from roots to above-gro<strong>und</strong> portions and release<br />
to the atmosphere has been elucidated by Nouchi et al.<br />
(1990), Butterbach-Bahl (1992), Nouchi and Mariko<br />
(1993), and Wang et al. (1997b). First, the dissolved<br />
CH 4 <strong>in</strong> soil water surro<strong>und</strong><strong>in</strong>g the roots diffuses <strong>in</strong>to<br />
the surface water <strong>of</strong> the roots and <strong>in</strong>to the cell-wall water<br />
<strong>of</strong> the root cortex (Fig. 1). This transfer is driven by<br />
the concentration gradient between the soil water surro<strong>und</strong><strong>in</strong>g<br />
the roots and the lysigenous <strong>in</strong>tercellular<br />
spaces <strong>in</strong> the roots. Butterbach-Bahl (1992) identified<br />
the cracks <strong>in</strong> the junction po<strong>in</strong>t <strong>of</strong> the ma<strong>in</strong> root and<br />
the root hairs as predom<strong>in</strong>ant entrance ports for CH 4<br />
from surro<strong>und</strong><strong>in</strong>g soil solution to the aerenchyma. CH 4<br />
is then gasified <strong>in</strong> the root cortex and transported to the
25<br />
shoots via the lysigenous <strong>in</strong>tercellular spaces and aerenchyma.<br />
Eventually, CH 4 is released to the atmosphere<br />
from various parts <strong>of</strong> the <strong>rice</strong> plant (Fig. 1). Nouchi et<br />
al. (1990) and Nouchi and Mariko (1993) observed that<br />
CH 4 is released ma<strong>in</strong>ly through micropores <strong>in</strong> the leaf<br />
sheath <strong>in</strong> the lower leaf but not from stomata. <strong>The</strong>y demonstrated<br />
that the closure <strong>of</strong> stomata open<strong>in</strong>gs by application<br />
<strong>of</strong> abscisic acid did not affect the CH 4 emission<br />
rate although the transpiration rate was decreased<br />
to one-third and stomatal resistance <strong>in</strong>creased threefold.<br />
However, more recently, with the use <strong>of</strong> 13 C-<br />
labeled CH 4 , Chanton et al. (1997) demonstrated that<br />
although CH 4 is transported by the <strong>rice</strong> plant predom<strong>in</strong>antly<br />
via molecular diffusion, a small component is<br />
also due to a transpiration-<strong>in</strong>duced flow.<br />
Most <strong>of</strong> CH 4 release is channelled through the culm<br />
(Nouchi and Mariko 1993) which is an aggregation <strong>of</strong><br />
leaf sheaths. In submerged <strong>rice</strong> <strong>plants</strong>, many air bubbles<br />
are released from (1) the abaxial epidermis <strong>of</strong> the<br />
leaf sheath and (2) near the junction <strong>of</strong> the nodal plate<br />
and leaf sheath (Nouchi and Mariko 1993). Wang et al.<br />
(1997b) observed a shift <strong>in</strong> the transport pathway with<br />
plant growth; about 50% <strong>of</strong> the CH 4 was released from<br />
leaf blades before shoot elongation whereas only a<br />
small amount was emitted through leaves as <strong>plants</strong><br />
grew older. In addition to the presence <strong>of</strong> micropores<br />
on the leaf sheath, Wang et al. (1997b) identified cracks<br />
at the junction <strong>of</strong> <strong>in</strong>ternodes. Although CH 4 can also be<br />
released from panicles, this pathway was negligible as<br />
long as leaves and nodes were not submerged. When<br />
the vegetative parts <strong>of</strong> the <strong>plants</strong> are submerged, the<br />
number <strong>of</strong> panicles determ<strong>in</strong>es the rate <strong>of</strong> CH 4 emission<br />
(Wang et al. 1997b).<br />
Factors controll<strong>in</strong>g CH 4 transfer rates through the <strong>rice</strong><br />
plant<br />
<strong>The</strong> actual flux <strong>of</strong> CH 4 through a <strong>rice</strong> plant depends on<br />
several factors such as the concentration <strong>of</strong> CH 4 <strong>in</strong> soil<br />
water, plant growth, size and shape, and <strong>rice</strong> cultivar.<br />
Nouchi and Mariko (1993) observed a l<strong>in</strong>ear relationship<br />
between CH 4 concentrations <strong>in</strong> the culture solutions<br />
and CH 4 emission rate from <strong>rice</strong> <strong>plants</strong>. CH 4 concentrations<br />
<strong>in</strong> <strong>rice</strong> <strong>plants</strong> show a clear gradient. <strong>The</strong><br />
highest concentrations are <strong>of</strong>ten fo<strong>und</strong> <strong>in</strong> the aerenchyma<br />
below the water level and highest CH 4 emissions<br />
occur through open<strong>in</strong>gs immediately above the water<br />
level (Wang et al. 1997b). <strong>The</strong> transport capacity <strong>of</strong> <strong>rice</strong><br />
<strong>plants</strong> also depends on the size and shape <strong>of</strong> <strong>plants</strong>.<br />
Emission rates from <strong>rice</strong> <strong>plants</strong> with n<strong>in</strong>e tillers were<br />
much larger than those with three tillers while the gap<br />
between flux rates widened with <strong>in</strong>creas<strong>in</strong>g CH 4 concentration<br />
<strong>in</strong> the soil (Nouchi and Mariko 1993). A similar<br />
relationship was fo<strong>und</strong> for leaf area at the tiller<strong>in</strong>g<br />
stage when nodes were not yet well developed (Wang<br />
et al. 1997b).<br />
Cutt<strong>in</strong>g <strong>of</strong>f the stems <strong>of</strong> <strong>rice</strong> <strong>plants</strong> above the floodwater<br />
did not <strong>in</strong>fluence CH 4 emission, <strong>in</strong>dicat<strong>in</strong>g that<br />
the rate-limit<strong>in</strong>g step <strong>in</strong> plant-mediated CH 4 transport<br />
was not located <strong>in</strong> the cut-<strong>of</strong>f part <strong>of</strong> the <strong>plants</strong> (Ando<br />
et al. 1983; Butterbach-Bahl 1992; Denier van der Gon<br />
and van Breemen 1993). In field experiments with cut<strong>of</strong>f<br />
stems, the pattern and magnitude <strong>of</strong> CH 4 emissions<br />
rema<strong>in</strong>ed unaffected over several days (<strong>Wassmann</strong> et<br />
al. 1994). Tracer gas experiments (Butterbach-Bahl et<br />
al. 1997) provided direct evidence that the root-shoot<br />
transition zone (Fig. 1b) is the ma<strong>in</strong> site <strong>of</strong> resistance to<br />
plant-mediated gas exchange between the soil and the<br />
atmosphere.<br />
Plant-mediated CH 4 transport does not depend on<br />
photosynthetic rates. Ando et al. (1983) demonstrated<br />
that darken<strong>in</strong>g <strong>of</strong> the <strong>plants</strong> or <strong>in</strong>creas<strong>in</strong>g CO 2 concentration<br />
<strong>in</strong> the atmosphere did not significantly affect the<br />
CH 4 emission rates. Partial submergence <strong>of</strong> stems and<br />
leaves could temporarily reduce the plant-mediated<br />
CH 4 emission while the flux rates readjust with<strong>in</strong> a few<br />
hours (Wang et al. 1997b). From these studies it appears<br />
that once the CH 4 is diffused <strong>in</strong>to the root aerenchyma<br />
and passes through the stem root <strong>in</strong>terception, it<br />
can escape to the atmosphere through one or the other<br />
non-submerged part <strong>of</strong> the <strong>rice</strong> <strong>plants</strong>.<br />
Impact <strong>of</strong> different <strong>rice</strong> plant traits<br />
S<strong>in</strong>ce up to 90% <strong>of</strong> CH 4 released from a <strong>rice</strong> field dur<strong>in</strong>g<br />
a grow<strong>in</strong>g season could be emitted by <strong>rice</strong> plantmediated<br />
transport, cultivar-specific properties may<br />
have a strong impact on CH 4 emission. In a study with<br />
six <strong>rice</strong> varieties, semi-dwarf varieties evolved 36% less<br />
CH 4 than tall <strong>rice</strong> varieties (L<strong>in</strong>dau et al. 1995). Other<br />
<strong>in</strong>vestigations <strong>in</strong> <strong>rice</strong> fields <strong>of</strong> India (Parashar et al.<br />
1991, 1994; Adhya et al. 1994), Ch<strong>in</strong>a (L<strong>in</strong> 1993), Japan<br />
(Watanabe et al. 1995b), Italy (Butterbach-Bahl et al.<br />
1997), and Texas, USA (Sigren et al. 1997) have also<br />
<strong>in</strong>dicated differences <strong>in</strong> the rate <strong>of</strong> CH 4 emission between<br />
different varieties. <strong>The</strong>se differences <strong>in</strong> CH 4 flux<br />
rates could be attributed to differences <strong>in</strong> CH 4 production,<br />
oxidation and gas transport capacities <strong>of</strong> different<br />
cultivars. Recently Butterbach-Bahl et al. (1997) observed<br />
that two Italian <strong>rice</strong> varieties differed by<br />
24–31.5% <strong>in</strong> their CH 4 emission dur<strong>in</strong>g two seasons.<br />
This relative difference which was observed irrespective<br />
<strong>of</strong> fertilizer treatment was not related to any difference<br />
<strong>in</strong> CH 4 production or oxidation, but was attributed<br />
to the different transfer capacities <strong>of</strong> the two cultivars.<br />
High transfer capacity co<strong>in</strong>cided with an <strong>in</strong>crease <strong>in</strong> the<br />
relative pore diameter <strong>of</strong> the root-shoot transition zone<br />
<strong>of</strong> the aerenchyma system (Butterbach-Bahl et al.<br />
1997).<br />
Many measurements <strong>of</strong> CH 4 emission <strong>in</strong> <strong>rice</strong> fields<br />
revealed seasonal patterns and variations <strong>in</strong> time and<br />
space. Seasonal CH 4 emission patterns from <strong>rice</strong> fields<br />
are the net result <strong>of</strong> the comb<strong>in</strong>ation <strong>of</strong> many factors<br />
such as reduc<strong>in</strong>g capacity <strong>of</strong> the soil, C source, nutrient<br />
level, <strong>rice</strong> plant, temperature, and agricultural practices.<br />
In a recent study, Wang et al. (1997c) observed
26<br />
similar emission patterns for the cultivars Dular and IR<br />
72 with one peak <strong>in</strong> the early and one <strong>in</strong> the late stage,<br />
whereas the cultivar IR 65598 did not develop a second<br />
emission peak. <strong>The</strong> dist<strong>in</strong>ct features <strong>of</strong> the new plant<br />
type IR 65598 were low organic C <strong>in</strong> root exudates,<br />
high oxidation power, few tillers and low dry matter per<br />
plant. However, these f<strong>in</strong>d<strong>in</strong>gs (obta<strong>in</strong>ed with <strong>in</strong>dividual<br />
<strong>plants</strong>) have to be confirmed for the situation encountered<br />
<strong>in</strong> the field.<br />
Relative importance <strong>of</strong> CH 4 transport pathways<br />
<strong>The</strong> plant-mediated flux <strong>in</strong>creases rapidly with<strong>in</strong> the<br />
first month after plant<strong>in</strong>g, which is also reflected <strong>in</strong> the<br />
relative contribution <strong>of</strong> this pathway to total emission<br />
(Table 1). In Italian <strong>rice</strong> fields, the aerenchyma transport<br />
contributed 88–90% <strong>of</strong> the overall emission<br />
throughout the reproductive and ripen<strong>in</strong>g stage (Butterbach-Bahl<br />
et al. 1997). Over the entire season, plantmediated<br />
transport can account for up to 90% <strong>of</strong> the<br />
total CH 4 emission (Cicerone and Shetter 1981; Holzapfel-Pschorn<br />
et al. 1985, 1986; Schütz et al. 1989; Butterbach-Bahl<br />
et al. 1997). However, the relative contribution<br />
<strong>of</strong> plant-mediated transfer is much lower <strong>und</strong>er<br />
high organic <strong>in</strong>puts (<strong>Wassmann</strong> et al. 1996). Organic<br />
amendments result <strong>in</strong> high ebullitive fluxes dur<strong>in</strong>g the<br />
first few weeks when <strong>plants</strong> are very young and have<br />
not developed their CH 4 transport mechanism. As a<br />
consequence, the relative significance <strong>of</strong> plant-mediated<br />
transport is decreased to values rang<strong>in</strong>g from<br />
48% to 85% (Table 1).<br />
Conclusions and future research needs<br />
<strong>The</strong> <strong>role</strong> <strong>of</strong> <strong>rice</strong> <strong>plants</strong> <strong>in</strong> the regulation <strong>of</strong> CH 4 emission<br />
comprises many facets <strong>in</strong>volv<strong>in</strong>g virtually every<br />
level <strong>of</strong> the CH 4 budget <strong>in</strong> <strong>rice</strong> fields. However, it<br />
should be noted that most <strong>of</strong> the f<strong>in</strong>d<strong>in</strong>gs on the effects<br />
<strong>of</strong> <strong>plants</strong> on CH 4 production and oxidation are derived<br />
<strong>in</strong>directly from emission rates. With the advent <strong>of</strong> techniques<br />
for <strong>in</strong>hibit<strong>in</strong>g CH 4 oxidation, future studies<br />
should address the <strong>in</strong>fluence <strong>of</strong> different plant parameters<br />
on CH 4 production and oxidation. Such <strong>in</strong>formation<br />
could be useful for formulat<strong>in</strong>g mitigation options<br />
and for future <strong>rice</strong> breed<strong>in</strong>g programs.<br />
One <strong>in</strong>dividual trait <strong>of</strong> the <strong>rice</strong> plant can represent<br />
an enhanc<strong>in</strong>g as well as a decreas<strong>in</strong>g factor for CH 4 emission.<br />
A high diffusion capacity <strong>of</strong> the aerenchyma entails<br />
higher efflux <strong>of</strong> CH 4 plus higher oxidation power<br />
<strong>of</strong> the root. <strong>The</strong>refore, it is imperative to balance the<br />
different impact <strong>mechanisms</strong> <strong>of</strong> <strong>rice</strong> <strong>plants</strong> for obta<strong>in</strong><strong>in</strong>g<br />
a holistic view <strong>of</strong> the CH 4 budget <strong>in</strong> <strong>rice</strong> fields. Recent<br />
achievements have considerably improved our <strong>und</strong>erstand<strong>in</strong>g<br />
the three decisive functions <strong>of</strong> <strong>plants</strong>, i.e.<br />
(1) root exudation, (2) CH 4 transport to the atmosphere<br />
and (3) CH 4 oxidation <strong>in</strong> the rhizosphere. But we<br />
are still far away from a comprehensive <strong>und</strong>erstand<strong>in</strong>g<br />
<strong>of</strong> these phenomena, especially with respect to the <strong>in</strong>teractive<br />
relationships between the <strong>mechanisms</strong> <strong>in</strong>volved.<br />
Consider<strong>in</strong>g the enormous variety <strong>in</strong> physiology and<br />
morphology <strong>of</strong> the genus Oryza, the impact <strong>of</strong> different<br />
traits is most important for devis<strong>in</strong>g mitigation strategies.<br />
Recent f<strong>in</strong>d<strong>in</strong>gs provide evidence that the desired<br />
trait <strong>of</strong> a low emission potential can be reconciled with<br />
a high yield potential (Wang et al. 1997a). High root<br />
exudation rates represent a loss <strong>of</strong> assimilates for the<br />
<strong>rice</strong> <strong>plants</strong> and can therefore be detrimental to yields.<br />
However, <strong>in</strong>formation is lack<strong>in</strong>g on the chemical composition<br />
(e.g. am<strong>in</strong>o acids, sugar C, organic acids) <strong>of</strong><br />
root exudates <strong>of</strong> different <strong>rice</strong> cultivars and its variations<br />
<strong>in</strong> response to different management practices.<br />
Comparative studies on cultivars may yield decisive<br />
clues as to the identification <strong>of</strong> favourable traits for mitigation<br />
and – at the same time – to improve our <strong>und</strong>erstand<strong>in</strong>g<br />
<strong>of</strong> the various functions <strong>of</strong> <strong>plants</strong> <strong>in</strong> the CH 4<br />
budget <strong>of</strong> <strong>rice</strong> fields. Whereas other mitigation strategies<br />
such as <strong>in</strong>termittent dra<strong>in</strong>age require substantial<br />
changes <strong>in</strong> farmers’ practices, new varieties can be <strong>in</strong>troduced<br />
with<strong>in</strong> a given sett<strong>in</strong>g. <strong>The</strong> identification <strong>of</strong><br />
cultivars that comb<strong>in</strong>e low emissions with high yields is<br />
the most challeng<strong>in</strong>g, but probably also the most promis<strong>in</strong>g,<br />
strategy for a susta<strong>in</strong>ed reduction <strong>of</strong> CH 4 emissions<br />
from <strong>rice</strong> fields.<br />
Table 1 Contribution <strong>of</strong> plant-mediated CH 4 emission at different sites and <strong>und</strong>er different treatments<br />
Site/country Fertilizer/Cultivar Plant<br />
age or<br />
<strong>in</strong>terval<br />
Overall CH 4<br />
emission rate<br />
(mg CH 4 m P2 h P1 )<br />
Plant-mediated<br />
CH 4 emission<br />
(% <strong>of</strong> overall<br />
emission)<br />
Source<br />
Vercelli/Italy Urea/Roma 25 days 7.8 0 Schütz et al. (1989)<br />
Urea/Roma 54 days 17.0 48<br />
Urea/Roma 76–103 days 23–28 90–97<br />
Vercelli/Italy Unfertilized/Roma S<strong>in</strong>gle season 11 88 Butterbach-Bahl (1992)<br />
Unfertilized/Lido S<strong>in</strong>gle season 8.1 90<br />
Los Baños/Philipp<strong>in</strong>es Urea/IR72 Dry season 1.1 85 <strong>Wassmann</strong> et al. (1996)<br />
Straw/IR72 Dry season 9.4 65<br />
Urea/IR72 Wet season 1.3 82<br />
Straw/IR72 Wet season 6.3 48
27<br />
Acknowledgement <strong>The</strong> review was conducted as part <strong>of</strong> the project<br />
“Reduction <strong>of</strong> CH 4 emission from <strong>rice</strong> fields by screen<strong>in</strong>g for<br />
low CH 4 transport capacity” f<strong>und</strong>ed by the German BMZ/GTZ<br />
(Project No. 95.7860.0–001.05)<br />
References<br />
Adhya TK, Rath AK, Gupta PK, Rao VR, Das SN, Parida KM,<br />
Parashar DC, Sethunathan N (1994) Methane emission from<br />
flooded <strong>rice</strong> fields <strong>und</strong>er irrigated conditions. Biol Fertil Soils<br />
18:245–248<br />
Andal R, Bhuvanesware K, Subba-Rao N (1956) Root exudates<br />
<strong>of</strong> paddy. Nature 178:1063<br />
Ando T, Yoshida S, Nishiyama I (1983) Nature <strong>of</strong> oxidiz<strong>in</strong>g power<br />
<strong>of</strong> <strong>rice</strong> roots. Plant Soil 72:57–71<br />
<strong>Aulakh</strong> MS, Doran JW, Mosier AR (1992) Soil denitrification –<br />
significance, measurement, and effects <strong>of</strong> management. Adv<br />
Soil Sci 18:1–57<br />
Armstrong W (1978) Root aeration <strong>in</strong> the wetland condition. In:<br />
Hook DD, Grawford RMM (eds) Plant life <strong>in</strong> anaerobic environments.<br />
Ann Arbor Press, Ann Arbor, Mich., pp 269–297<br />
Armstrong W (1979) Aeration <strong>in</strong> higher <strong>plants</strong>. Adv Bot Res<br />
7:225–332<br />
Barber DA, Ebert M, Sevans NT (1962) <strong>The</strong> movement <strong>of</strong> 15 O 2<br />
through barley and <strong>rice</strong> <strong>plants</strong>. J Exp Bot 13:397–403<br />
Bedard C, Knowles R (1989) Physiology, biochemistry and specific<br />
<strong>in</strong>hibitors <strong>of</strong> CH 4 , NH 4 and CO oxidation by methanotrophs<br />
and nitrifiers. Microbiol Rev 53 :68–84<br />
Bhadrachalam A, Chakravorti SP, Banerjee NK, Mohanty SK,<br />
Mosier AR (1992) Denitrification <strong>in</strong> <strong>in</strong>termittently flooded<br />
<strong>rice</strong> fields and N-gas transport through <strong>rice</strong> <strong>plants</strong>. Ecol Bull<br />
(Stockh) 42:183–187<br />
Butterbach-Bahl K (1992) Mechanismen der Produktion <strong>und</strong> Emission<br />
von Methan <strong>in</strong> Reisfeldern: Abhängigkeit von Felddüngung<br />
<strong>und</strong> angebauter Varietät. Dissertation, Technical University<br />
<strong>of</strong> Munich. Schriftenreihe des Fraunh<strong>of</strong>er Institut für<br />
Atmospherische Umweltforschung, vol 14. Mauraun, Frankfurt/M<br />
Butterbach-Bahl K, Papen H, Rennenberg H (1997) Impact <strong>of</strong><br />
gas transport through <strong>rice</strong> cultivars on methane emission from<br />
paddy fields. Plant Cell Environ 20:1175–1183<br />
Chanton JP, Whit<strong>in</strong>g GJ, Blair NE, L<strong>in</strong>dau CW, Bollich PK<br />
(1997) Methane emission from <strong>rice</strong>: stable isotopes, diurnal<br />
variations, and CO 2 exchange. Global Biogeochem Cycles<br />
11:15–27<br />
Chidthaisong A, Watanabe I (1997a) Changes <strong>in</strong> concentration<br />
and d 13 C values <strong>of</strong> soil entrapped CH 4 and CO 2 <strong>in</strong> flooded<br />
<strong>rice</strong> soil. Biol Fertil Soils 24:70–75<br />
Chidthaisong A, Watanabe I (1997b). Methane formation and<br />
emission from <strong>rice</strong> soil <strong>in</strong>corporated with 13 C-labeled <strong>rice</strong><br />
straw. Soil Biol Biochem 29:1173–1181<br />
Cicerone RJ, Shetter JD (1981) Sources <strong>of</strong> atmospheric methane:<br />
measurements <strong>in</strong> <strong>rice</strong> paddies and a discussion. J Geophys Res<br />
86:7203–7209<br />
Cicerone RJ, Shetter JD, Delwiche CC (1983) Seasonal variations<br />
<strong>of</strong> methane flux from a California <strong>rice</strong> paddy. J Geophys Res<br />
88:7203–7209<br />
Conrad R, Rothfuss F (1991) Methane oxidation <strong>in</strong> the soil surface<br />
layer <strong>of</strong> a flooded <strong>rice</strong> field and the effect <strong>of</strong> ammonium.<br />
Biol Fertil Soils 12 :28–32<br />
De Bont JAM, Lee KK, Bould<strong>in</strong> DF (1978) Bacterial oxidation <strong>of</strong><br />
methane <strong>in</strong> a <strong>rice</strong> paddy. Ecol Bull (Stockh) 26 :91–96<br />
Denier van der Gon HAC, Breemen N van (1993) Diffusion-controlled<br />
transport <strong>of</strong> methane from soil to atmosphere as mediated<br />
by <strong>rice</strong> <strong>plants</strong>. Biogeochemistry 21:177–190<br />
Denier van der Gon HAC, Neue HU (1996) Oxidation <strong>of</strong> methane<br />
<strong>in</strong> the rhizosphere <strong>of</strong> <strong>rice</strong> <strong>plants</strong>. Biol Fertil Soils<br />
22:359–366<br />
Denier van der Gon HAC, Neue HU, Lant<strong>in</strong> RS, <strong>Wassmann</strong> R,<br />
Alberto MRC, Aduna JB, Tan MJP (1992) Controll<strong>in</strong>g factors<br />
<strong>of</strong> methane emission from <strong>rice</strong> fields. In: Batjes NH, Bridges<br />
EM (eds) World <strong>in</strong>ventory <strong>of</strong> soil emission potentials: WISE<br />
report no 2. International Soil Reference and Information<br />
Centre, Wagen<strong>in</strong>gen, pp 81–92<br />
Dlugokencky EJ, Masarie KA, Lang PM, Tans PP (1998) Cont<strong>in</strong>u<strong>in</strong>g<br />
decl<strong>in</strong>e <strong>in</strong> the growth rate <strong>of</strong> the atmospheric methane<br />
burden. Nature 393:447–450<br />
Epp MA, Chanton JP (1993) Rhizospheric methane oxidation determ<strong>in</strong>ed<br />
via methyl fluoride <strong>in</strong>hibition technique. J Geophys<br />
Res 98:18413–18422<br />
Frenzel P, Rothfuss F, Conrad R (1992) Oxygen pr<strong>of</strong>iles and methane<br />
turnover <strong>in</strong> a flooded <strong>rice</strong> microcosm. Biol Fertil Soils<br />
14:84–89<br />
Gambrell RP, Patrick WH Jr (1978) Chemical and microbiological<br />
properties <strong>of</strong> anaerobic soils and sediments. In: Hook DD,<br />
Grawford RMM (eds) Plant life <strong>in</strong> anaerobic environments.<br />
Ann Arbor Press, Ann Arbor, Mich., pp 375–423<br />
Godo GH, Reisenauer HM (1980) Plant effects on soil manganese<br />
availability. Soil Sci Soc Am J 44 :993–995<br />
Grosse W, Schroeder P (1985) Aeration <strong>of</strong> the roots and chloroplast-free<br />
tissues <strong>of</strong> trees. Ber Dtsch Bot Ges 98:311–318<br />
Higudchi T (1982) Gaseous CO 2 transport through aerenchyma<br />
and <strong>in</strong>tercellular spaces <strong>in</strong> relation to the uptake <strong>of</strong> CO 2 by<br />
<strong>rice</strong> roots. Soil Sci Plant Nutr 28:491–497<br />
Higudchi T, Yoda K, Tensho K (1984) Further evidence for gaseous<br />
CO 2 transport <strong>in</strong> relation to root uptake <strong>of</strong> CO 2 <strong>in</strong> <strong>rice</strong><br />
plant. Soil Sci Plant Nutr (Tokyo) 30 :125–136<br />
H<strong>of</strong>fland E, F<strong>in</strong>denegg GR, Nelemans JA (1989) Solubilization<br />
<strong>of</strong> rock phosphate by rape. II. Local root exudation <strong>of</strong> organic<br />
acids as a response to P-starvation. Plant Soil 113 :161–165<br />
Holzapfel-Pschorn A, Seiler W (1986) Methane emission dur<strong>in</strong>g a<br />
cultivation period from an Italian <strong>rice</strong> paddy. J Geophy Res<br />
91:11803–11814<br />
Holzapfel-Pschorn A, Conrad R, Seiler W (1985) Production, oxidation<br />
and emission <strong>of</strong> methane <strong>in</strong> <strong>rice</strong> paddies. FEMS Microbiol<br />
Ecol 31:345–351<br />
Holzapfel-Pschorn A, Conrad R, Seiler W (1986) Effects <strong>of</strong> vegetation<br />
on the emission <strong>of</strong> methane from submerged paddy soil.<br />
Plant Soil 92:223–391<br />
Horst WJ, Wagner A, Marschner H (1982) Mucilage protects root<br />
meristems from alum<strong>in</strong>ium <strong>in</strong>jury. Z Pflanzenphysiol<br />
105:435–444<br />
Horst WJ, Klotz F, Szulkiewiz P (1990) Mechanical impedance<br />
<strong>in</strong>creases alum<strong>in</strong>ium tolerance <strong>of</strong> soybean (Glyc<strong>in</strong>e max)<br />
roots. Plant Soil 124 :227–231<br />
Inubushi K, Muramatsu Y, Umerayasi M (1992) Influence <strong>of</strong> percolation<br />
on methane emission from flooded paddy soil. Jpn J<br />
Soil Sci Plant Nutr 63:184–189<br />
IPPC (1992). Climate Change. In: Houghton JT, Callander BA,<br />
Varney SK (eds) <strong>The</strong> Supplementary Report to the IPCC<br />
Scientific Assessment. Intergovernmental Panel on Climate<br />
Change, Cambridge University Press, Cambridge<br />
IPPC (1994) Climate change. In: Houghton JT, et al (ed) Radiative<br />
forc<strong>in</strong>g <strong>of</strong> climate change and an evaluation <strong>of</strong> the Intergovernmental<br />
Panel on Climate Change IS92. Emission scenarios.<br />
Cambridge University Press, Cambridge. 364 pp<br />
Jauregui MA, Reisenauer HM (1982) Dissolution <strong>of</strong> oxides manganese<br />
and iron by root exudate components. Soil Sci Soc Am<br />
J 46:314–317<br />
Keeley JE (1979) Population differentiation along a flood frequency<br />
gradient: physiological adaptations to flood<strong>in</strong>g <strong>in</strong> Nyssa<br />
sylvatica. Ecol Monogr 49 :89–108<br />
K<strong>in</strong>g GM (1992) Ecological aspects <strong>of</strong> methane oxidation, a key<br />
determ<strong>in</strong>ant <strong>of</strong> global methane dynamics. Adv Microbial Ecol<br />
12:431–468<br />
Kludze HK, DeLaune RD, Patrick WH Jr (1993) Aerenchyma<br />
formation and methane and oxygen exchange <strong>in</strong> <strong>rice</strong>. Soil Sci<br />
Soc Am J 57:386–391
28<br />
Kludze HK, Pezeshki SR, DeLaune RD (1994) Evaluation <strong>of</strong><br />
root oxygenation and growth <strong>in</strong> baldcypress <strong>in</strong> response to<br />
short-term soil hypoxia. Can J For Res 24 :1–5<br />
Knowles R (1993) Methane: Processes <strong>of</strong> production and consumption.<br />
In: Agricultural ecosystem effects on trace gases<br />
and global climate change. Special Publication 55. Am Soc<br />
Agron, Madison, Wis., pp 145–155<br />
L<strong>in</strong> E (1993) Agricultural techniques: factors controll<strong>in</strong>g methane<br />
emissions. In: Gao L, Wu L, Zheng D, Ham X (eds) Proceed<strong>in</strong>gs<br />
<strong>of</strong> the International Symposium on Climate Change, Natural<br />
Disasters, and Agricultural Strategies. Ch<strong>in</strong>a Meteorol.<br />
Press, Beij<strong>in</strong>g, pp 120–126<br />
L<strong>in</strong>, M, You C (1989) Root exudates <strong>of</strong> <strong>rice</strong> (Oryza sativa L) and<br />
its <strong>in</strong>teraction with Alcaligenes faecalis (<strong>in</strong> Ch<strong>in</strong>ese). Sci Agric<br />
S<strong>in</strong> 22 :6–12<br />
L<strong>in</strong>dau CW, Bollich PK, DeLaune RD, Patrick WH Jr, Law VJ<br />
(1991) Effect <strong>of</strong> urea fertilizer and environmental factors on<br />
CH 4 emissions from a Louisiana USA, <strong>rice</strong> field. Plant Soil<br />
136:195–203<br />
L<strong>in</strong>dau CW, Bollich PK, DeLaune RD (1995) Effect <strong>of</strong> <strong>rice</strong> variety<br />
on methane emission from Louisiana <strong>rice</strong>. Agric Ecosyst<br />
Environ 54:109–114<br />
MacRae IC, Castro TF (1966) Carbohydrates and am<strong>in</strong>o acids <strong>in</strong><br />
the root exudates <strong>of</strong> <strong>rice</strong> seedl<strong>in</strong>gs. Phyton 23:95–100<br />
Marschner H (1995) M<strong>in</strong>eral nutrition <strong>of</strong> higher <strong>plants</strong>, 2nd edn.<br />
Academic Press, New York<br />
Mosier AR, Mohanty SK, Bhadrachalam A, Chakravorti SP<br />
(1990) Evolution <strong>of</strong> d<strong>in</strong>itrogen and nitrous oxide from the soil<br />
to the atmosphere through <strong>rice</strong> <strong>plants</strong>. Biol Fertil Soils<br />
9:61–67<br />
Munch JC, Ottow JCG (1977) Modelluntersuchungen zum Mechanismus<br />
der bakteriellen Eisenreduktionen <strong>in</strong> hydromorphen<br />
Böden. Z Pflanzenernähr Bodenkd 140 :549–562<br />
Munch JC, Ottow JCG (1980) Preferential reduction <strong>of</strong> amorphous<br />
to crystall<strong>in</strong>e iron oxides by bacterial activity. Soil Sci<br />
129:15–21<br />
Munch JC, Ottow JCG (1983) Reductive transformation mechanism<br />
<strong>of</strong> ferric oxides <strong>in</strong> hydromorphic soils. Ecol Bull (Stockh)<br />
35:383–394<br />
Nagarajah S, Posner AM, Quirk JP (1970) Competitive adsorption<br />
<strong>of</strong> phosphate with polygalacturonate and other organic<br />
anions on kaol<strong>in</strong>ite and oxide surfaces. Nature 228:83–84<br />
Nagarajan R, Prasad NN (1983) Biochemical aspects <strong>of</strong> seed –<br />
exudates <strong>of</strong> <strong>rice</strong>. Sci Cult 49:58–59<br />
Nambiar EKS (1976) <strong>The</strong> uptake <strong>of</strong> z<strong>in</strong>c-65 by roots <strong>in</strong> relation to<br />
soil water content and root growth. Aust J Soil Res<br />
14:67–74<br />
Neue HU, Sass RL (1998) <strong>The</strong> budget <strong>of</strong> methane from <strong>rice</strong><br />
fields. IG ACtiv 17:3–11<br />
Neue HU, <strong>Wassmann</strong> R, Kludze HK, Wang B, Lant<strong>in</strong> RL (1997)<br />
Factors and processes controll<strong>in</strong>g methane emissions from <strong>rice</strong><br />
fields. Nutr Cycl<strong>in</strong>g Agroeco 49 :111–117<br />
Nouchi I, Mariko S (1993) Mechanism <strong>of</strong> methane transport by<br />
<strong>rice</strong> <strong>plants</strong>. In: Oremland RS (ed) Biogeochemistry <strong>of</strong> global<br />
change. Chapman & Hall, New York, pp 336–352<br />
Nouchi I, Mariko S, Aoki K (1990) Mechanism <strong>of</strong> methane transport<br />
from the rhizosphere to the atmosphere through <strong>rice</strong><br />
<strong>plants</strong>. Plant Physiol 94:59–66<br />
Oremland RS, Culbertson CW (1992a) Evaluation <strong>of</strong> methyl fluoride<br />
and dimethyl ether as <strong>in</strong>hibitors <strong>of</strong> aerobic methane oxidation.<br />
Appl Environ Microbiol 58:2983–2992<br />
Oremland RS, Culbertson CW (1992b) Importance <strong>of</strong> methaneoxidiz<strong>in</strong>g<br />
bacteria <strong>in</strong> the methane budget as revealed by the<br />
use <strong>of</strong> a specific <strong>in</strong>hibitor. Nature 356:421–423<br />
Oremland RS, Taylor BF (1995) Inhibition <strong>of</strong> methogenesis <strong>in</strong><br />
mar<strong>in</strong>e sediments by acetylene and ethylene: Validity <strong>of</strong> the<br />
acetylene reduction assay for anaerobic microcosms. Appl Microbiol<br />
30:707–709<br />
Ottow JCW (1969) Distribution and differentiation <strong>of</strong> iron-reduc<strong>in</strong>g<br />
bacteria <strong>in</strong> gley soils. Zentralbl Bakteriol Abt II<br />
123:600–615<br />
Ottow JCW, Munch JC (1978) Mechanisms <strong>of</strong> reductive transformations<br />
<strong>in</strong> the anaerobic microenvironment <strong>of</strong> hydromorphic<br />
soils. In: Krumbe<strong>in</strong> WE (ed) Environmental biogeochemistry<br />
and geomicrobiology, vol 2. Ann Arbor Science Publishers,<br />
Ann Arbor, Mich., pp 483–491<br />
Papen H, Rennenberg H (1990) Microbial processes <strong>in</strong>volved <strong>in</strong><br />
the emission <strong>of</strong> radioactively important trace gases. In: Trans<br />
14th Int Soil Sci Congr, vol 2. Kyoto, pp 232–237<br />
Parashar DC, Rai J, Gupta PK, S<strong>in</strong>gh N (1991) Parameters affect<strong>in</strong>g<br />
methane emission from paddy fields. Indian J Radio Space<br />
Phys 20 :12–17<br />
Parashar DC, et al (1994) Methane budget from Indian paddy<br />
fields. In: M<strong>in</strong>ami K, Mosier A, Sass RL (eds) CH 4 and N 2 O<br />
global emissions and controls from <strong>rice</strong> fields and other agricultural<br />
and <strong>in</strong>dustrial sources. NIAES Series 2. National Institute<br />
<strong>of</strong> Agro-Environmental Sciences, Tsukuba, Japan, pp<br />
27–39<br />
Ponnamperuma FN (1972) <strong>The</strong> chemistry <strong>of</strong> submerged soils.<br />
Adv Agron 24:29–96<br />
Prade K, Trolldenier G (1990) Denitrification <strong>in</strong> the rhizosphere<br />
<strong>of</strong> <strong>plants</strong> with <strong>in</strong>herently different aerenchyma formation:<br />
wheat (Triticum aestivum) and <strong>rice</strong> (Oryza sativa). Biol Fertil<br />
Soils 9:215–219<br />
Raimbault M, R<strong>in</strong>audo G, Garcia JL, Boreau M (1977) A device<br />
to study metabolic gases <strong>in</strong> the rhizosphere. Soil Biol Biochem<br />
9:193–196<br />
Rask<strong>in</strong> I, Kende H (1985) Mechanism <strong>of</strong> aeration <strong>in</strong> <strong>rice</strong>. Science<br />
228:327–329<br />
Rennenberg H, <strong>Wassmann</strong> R, Papen H, Seiler W (1992) Trace<br />
gas emission <strong>in</strong> <strong>rice</strong> cultivation. Ecol Bull (Stockh)<br />
42:164–173<br />
Sass RL, Fisher FM, Harcombe PA, Turner FT (1990) Methane<br />
production and emission <strong>in</strong> a Texas <strong>rice</strong> field. Global Biogeochem<br />
Cycles 4:47–68<br />
Sass RL, Fisher FM, Harcombe PA, Turner FT (1991a). Mitigation<br />
<strong>of</strong> methane emissions from <strong>rice</strong> fields: possible adverse<br />
effects <strong>of</strong> <strong>in</strong>corporated <strong>rice</strong> straw. Global Biogeochem Cycles<br />
5:275–287<br />
Sass RL, Fisher FM, Harcombe PA, Turner FT (1991b) Methane<br />
emission from <strong>rice</strong> fields as <strong>in</strong>fluenced by solar radiation, temperature<br />
and straw <strong>in</strong>corporation. Global Biogeochem Cycles<br />
5:335–350<br />
Schönwitz R, Ziegler H (1982) Exudation <strong>of</strong> water-soluble vitam<strong>in</strong>s<br />
and <strong>of</strong> some carbohydrates by <strong>in</strong>tact roots <strong>of</strong> maize seedl<strong>in</strong>gs<br />
(Zea mays L.) <strong>in</strong>to a m<strong>in</strong>eral nutrient solution. Z Pflanzenphysiol<br />
107:7–14<br />
Schütz H, Seiler W, Conrad R (1989) Processes <strong>in</strong>volved <strong>in</strong> formation<br />
and emission <strong>of</strong> methane <strong>in</strong> <strong>rice</strong> paddies. Biogeochemistry<br />
7:33–53<br />
Schütz H, Schroeder P, Rennenberg H (1991) Role <strong>of</strong> <strong>plants</strong> <strong>in</strong><br />
regulat<strong>in</strong>g the methane flux to the atmosphere. In: Sharkey<br />
TD, Holland EA, Mooney HA (eds) Trace gas emissions by<br />
<strong>plants</strong>. Academic Press, San Diego, pp 29–63<br />
Sebacher DI, Harriss RC, Bartlett KB (1985) Methane emissions<br />
to the atmosphere through aquatic <strong>plants</strong>. J Environ Qual<br />
14:40–46<br />
Seiler W, Holzapfel-Pschorn A, Conrad R, Scharffe D (1984) Methane<br />
emission from <strong>rice</strong> paddies. J Atmos Chem 1:241–268<br />
Sigren LK, Byrd GT, Fisher FM, Sass RL (1997) Comparison <strong>of</strong><br />
soil accetate concentrations and methane production, transport,<br />
and emission <strong>in</strong> two <strong>rice</strong> cultivars. Global Biochem Cycles<br />
11 :1–14<br />
Takai Y (1961) Reduction and microbial metabolism <strong>in</strong> paddy<br />
soils (3) (<strong>in</strong> Japanese). Nogyo Gijutsu 19:122–126<br />
Takai Y, Kamura T (1966) <strong>The</strong> mechanism <strong>of</strong> reduction <strong>in</strong> waterlogged<br />
paddy soils. Folia Microbiol 11:304–313<br />
Trolldenier G (1977) M<strong>in</strong>eral nutrition and reduction processes <strong>in</strong><br />
the rhizosphere <strong>of</strong> <strong>rice</strong>. Plant Soil 47:193–202<br />
Trolldenier G (1981) Influence <strong>of</strong> soil moisture, soil acidity and<br />
nitrogen source on take-all <strong>of</strong> wheat. Phytopathol Z<br />
102:217–222
29<br />
Ueckert J, Hurek T, Fendrik I, Niemann EG (1990) Radial gas<br />
diffusion from roots <strong>of</strong> <strong>rice</strong> and kallargrass (Leptochloa fusca<br />
L. Kunth), and effects <strong>of</strong> <strong>in</strong>oculation with Azospirillum brasilence<br />
Cd. Plant Soil 112:59–65<br />
Van Raalte MH (1941) On the oxygen supply <strong>of</strong> <strong>rice</strong> <strong>plants</strong>. Ann<br />
Bot Gard Buitenzorg 51:43–57<br />
Wang B (1995) Effect <strong>of</strong> <strong>rice</strong> cultivar on diel and seasonal methane<br />
emission. Ph D. <strong>The</strong>sis. University <strong>of</strong> Philipp<strong>in</strong>es, Los<br />
Baños, Philipp<strong>in</strong>es<br />
Wang B, Neue HU, Samonte HP (1997a) Effect <strong>of</strong> cultivar difference<br />
(‘IR72’, ‘IR65598’ and ‘Dular’) on methane emission.<br />
Agric Ecosyst Environ 62 :31–40<br />
Wang B, Neue HU, Samonte HP (1997b) Role <strong>of</strong> <strong>rice</strong> <strong>in</strong> mediat<strong>in</strong>g<br />
methane emission. Plant Soil 189:107–115<br />
Wang B, Neue HU, Samonte HP (1997c) Effect <strong>of</strong> <strong>rice</strong> plant on<br />
seasonal methane emission patterns. Acta Agron S<strong>in</strong><br />
23:271–279<br />
Wang ZP, Delaune RD, Masscheleyn PH, Patrick WH Jr (1993)<br />
Soil redox and pH effects on methane production <strong>in</strong> a flooded<br />
<strong>rice</strong> soil. Soil Sci Soc Am J 57:382–385<br />
<strong>Wassmann</strong> R, Papen H, Rennenberg H (1993a) Methane emission<br />
from <strong>rice</strong> paddies and possible mitigation options. Chemosphere<br />
26:201–217<br />
<strong>Wassmann</strong> R, Wang MX, Shangguan XJ, Xie XL, Shen RX, Papen<br />
H, Rennenberg H, Seiler W (1993b) First records <strong>of</strong> a<br />
field experiment on fertilizer effect on methane emission from<br />
<strong>rice</strong> fields <strong>in</strong> Human prov<strong>in</strong>ce (PR Ch<strong>in</strong>a) Geophys Res Lett<br />
20:2071–2074<br />
<strong>Wassmann</strong> R, Neue HU, Lant<strong>in</strong> RS, Aduna JB, Alberto MCR,<br />
Andales MJ, Tan MJ, Denier van der Gon HAC, H<strong>of</strong>fmann<br />
H, Papen H, Rennenberg H, Seiler W (1994) Temporal patterns<br />
<strong>of</strong> methane emissions from wetland <strong>rice</strong>fields treated by<br />
different modes <strong>of</strong> N application. J Geophys Res<br />
99:16457–16462<br />
<strong>Wassmann</strong> R, Neue HU, Alberto MCR, Lant<strong>in</strong> RS, Bueno C,<br />
Llenaresas D, Arah JRM, Papen H, Seiler W, Rennenberg H<br />
(1996) Fluxes and pools <strong>of</strong> methane <strong>in</strong> wetland <strong>rice</strong> soils with<br />
vary<strong>in</strong>g organic <strong>in</strong>puts. Environ Monitor Assess 42:163–173<br />
<strong>Wassmann</strong> R, Moya TB, Lant<strong>in</strong> RS (1998) Rice and global<br />
change. In: Dowl<strong>in</strong>g NG, Greenfield SM, Fischer KS (eds)<br />
Susta<strong>in</strong>ability <strong>of</strong> <strong>rice</strong> <strong>in</strong> the global food system. Pacific Bas<strong>in</strong><br />
Study Center and International Rice Research Institute, Manila,<br />
Philipp<strong>in</strong>es, pp 205–224<br />
Watanabe I (1984) Anaerobic decomposition <strong>of</strong> organic matter <strong>in</strong><br />
flooded <strong>rice</strong> soils. In: Organic matter and <strong>rice</strong>. International<br />
Rice Research Institute, Los Baños, Philipp<strong>in</strong>es, pp 237–258<br />
Watanabe I, Takada G, Hashimoto T, Inubushi K (1995a) Evaluation<br />
<strong>of</strong> alternative substrates for determ<strong>in</strong><strong>in</strong>g methane-oxidiz<strong>in</strong>g<br />
activities and methanotrophic populations <strong>in</strong> soils. Biol<br />
Fertil Soils 20:101–106<br />
Watanabe A, Kajiwara M, Tashiro T, Kimura M (1995b) Influence<br />
<strong>of</strong> <strong>rice</strong> cultivar on methane emission from paddy<br />
fields. Plant Soil 17 :51–56<br />
Watanabe I, Hashimoto T, Shimoyama A (1997) Methane-oxidiz<strong>in</strong>g<br />
activities and methanotrophic populations associated with<br />
wetland <strong>rice</strong> soils. Biol Fertil Soils 24 :261–265<br />
Yagi K, Chairoj P, Tsurata H, Cholitkul W, M<strong>in</strong>ami K (1994) Methane<br />
emission from Japanese and Thai paddy fields. In: M<strong>in</strong>ami<br />
K, Mosier A, Sass RL (eds) CH 4 and N 2 O global emissions<br />
and controls from <strong>rice</strong> fields and other agricultural and<br />
<strong>in</strong>dustrial sources. NIAES Series 2. National Institute <strong>of</strong><br />
Agro-Environmental Sciences, Tsukuba, Japan, pp 41–53<br />
Yagi K, M<strong>in</strong>ami K (1990) Effects <strong>of</strong> organic application on methane<br />
emission from Japanese paddy fields. In: Bouwman AF<br />
(ed) Soils and greenhouse effect. Wiley, Chichester, pp<br />
467–473<br />
Yoshida T (1975) Microbial metabolism <strong>in</strong> flooded soils. In: Paul<br />
EA, McLaren AD (eds) Soil biochemistry, vol 3. Dekker,<br />
New York, pp 83–122