A. Data Definition Tables
A. Data Definition Tables
A. Data Definition Tables
Sie wollen auch ein ePaper? Erhöhen Sie die Reichweite Ihrer Titel.
YUMPU macht aus Druck-PDFs automatisch weboptimierte ePaper, die Google liebt.
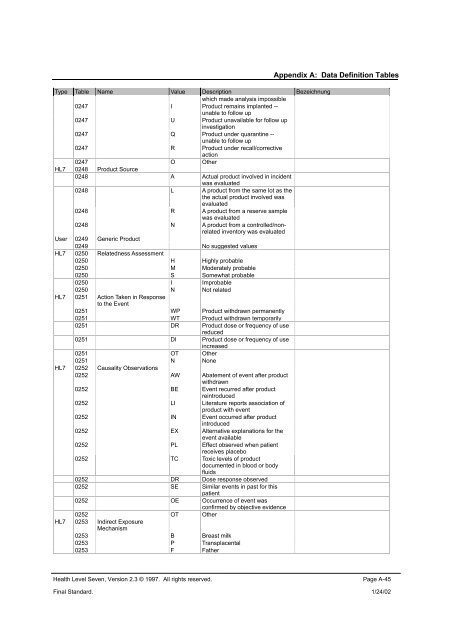
Type Table Name Value Description Bezeichnung<br />
which made analysis impossible<br />
0247 I Product remains implanted --<br />
unable to follow up<br />
0247 U Product unavailable for follow up<br />
investigation<br />
0247 Q Product under quarantine --<br />
unable to follow up<br />
0247 R Product under recall/corrective<br />
action<br />
0247 O Other<br />
HL7 0248 Product Source<br />
0248 A Actual product involved in incident<br />
was evaluated<br />
0248 L A product from the same lot as the<br />
the actual product involved was<br />
evaluated<br />
0248 R A product from a reserve sample<br />
was evaluated<br />
0248 N A product from a controlled/nonrelated<br />
inventory was evaluated<br />
User 0249 Generic Product<br />
0249 No suggested values<br />
HL7 0250 Relatedness Assessment<br />
0250 H Highly probable<br />
0250 M Moderately probable<br />
0250 S Somewhat probable<br />
0250 I Improbable<br />
0250 N Not related<br />
HL7 0251 Action Taken in Response<br />
to the Event<br />
0251 WP Product withdrawn permanently<br />
0251 WT Product withdrawn temporarily<br />
0251 DR Product dose or frequency of use<br />
reduced<br />
0251 DI Product dose or frequency of use<br />
increased<br />
0251 OT Other<br />
0251 N None<br />
HL7 0252 Causality Observations<br />
0252 AW Abatement of event after product<br />
withdrawn<br />
0252 BE Event recurred after product<br />
reintroduced<br />
0252 LI Literature reports association of<br />
product with event<br />
0252 IN Event occurred after product<br />
introduced<br />
0252 EX Alternative explanations for the<br />
event available<br />
0252 PL Effect observed when patient<br />
receives placebo<br />
0252 TC Toxic levels of product<br />
documented in blood or body<br />
fluids<br />
0252 DR Dose response observed<br />
0252 SE Similar events in past for this<br />
patient<br />
0252 OE Occurrence of event was<br />
confirmed by objective evidence<br />
0252 OT Other<br />
HL7 0253 Indirect Exposure<br />
Mechanism<br />
0253 B Breast milk<br />
0253 P Transplacental<br />
0253 F Father<br />
Appendix A: <strong>Data</strong> <strong>Definition</strong> <strong>Tables</strong><br />
Health Level Seven, Version 2.3 © 1997. All rights reserved. Page A-45<br />
Final Standard. 1/24/02