Herausforderungen an die Abgasreinigung von morgen - Deutsche ...
Herausforderungen an die Abgasreinigung von morgen - Deutsche ...
Herausforderungen an die Abgasreinigung von morgen - Deutsche ...
Sie wollen auch ein ePaper? Erhöhen Sie die Reichweite Ihrer Titel.
YUMPU macht aus Druck-PDFs automatisch weboptimierte ePaper, die Google liebt.
ASPEKTE<br />
identical) <strong>an</strong>d increases monotonically with increasing temperature.<br />
Whereas the interfacial tension �bc decreases<br />
(monotonically) with increasing temperature <strong>an</strong>d v<strong>an</strong>ishes<br />
at Tu, because the two phases (c) <strong>an</strong>d (b) become identical<br />
there. This opposite temperature dependence of �ac <strong>an</strong>d<br />
�bc results in a minimum in their sum, �ac + �bc. In order to<br />
assure the stability of the water/oil interface �ab � �ac + �bc<br />
must hold. Otherwise a thin layer of the middle-phase<br />
would penetrate between the water- <strong>an</strong>d oil-rich excess phase.<br />
Therefore �ab has to pass through a minimum within the<br />
three phase region, i.e. being more precisely at the phase<br />
inversion temperature.<br />
In conclusion, at the PIT the minimum in the water/oil interfacial<br />
tension enables the optimal solubilisation of water <strong>an</strong>d<br />
oil, i.e. with the minimum amount of surfact<strong>an</strong>t �. That this<br />
correlation between phase behaviour <strong>an</strong>d interfacial tensions<br />
holds also for technical applications was shown by the removal<br />
of hexadec<strong>an</strong>e from synthetic tissue, which reaches a<br />
maximum within the three phase region [41,42]. The value<br />
of the water/oil-interfacial tension �ab c<strong>an</strong> be estimated<br />
considering the simple argument from Volmer [43,44] that<br />
thermal energy will lead to dispersion as soon as the interfacial<br />
tension times the area of the object becomes smaller<br />
th<strong>an</strong> kT, i.e. �abr 2 ≈ kT.<br />
2.2 MICROSTRUCTURE<br />
Most of the recent applications of microemulsions depend<br />
on the fact that microemulsions, although macroscopically<br />
homogeneous, are heterogeneous on the sub-microscopically<br />
scale. Topologically ordered interfacial fi lms are formed<br />
by the surfact<strong>an</strong>t molecules which are forced into the microscopic<br />
water/oil interface because of their amphiphilicity.<br />
The nature <strong>an</strong>d properties of these microscopic interfacial<br />
fi lms are essential for the formations of microemulsions<br />
<strong>an</strong>d in particular for the most interesting feature of microemulsions,<br />
i.e. their microstructure. 50 years ago, Winsor<br />
[2] <strong>an</strong>d Schulm<strong>an</strong> [45] suggested that microemulsions<br />
are always spherical exhibit microstructures, <strong>an</strong>d that a<br />
layered, lamellar structure exists as the exception in the<br />
middle phase. In 1976 Scriven [46] put forward the crucial<br />
idea of the bicontinuous structure of the surfact<strong>an</strong>trich<br />
middle phase, which 10 years later was proven with<br />
the help of NMR self-diffusion measurements [25,26] <strong>an</strong>d<br />
the direct visualization by freeze fracture electron microscopy<br />
(FFEM) [25,27]. Further stu<strong>die</strong>s of the micro-structure<br />
by NMR-self diffusion, tr<strong>an</strong>smission electron microscopy<br />
(TEM) <strong>an</strong>d scattering techniques (SAXS <strong>an</strong>d SANS) proved<br />
structures droplet- <strong>an</strong>d wormlike micro, sample sp<strong>an</strong>ning<br />
networks <strong>an</strong>d bicontinuous structures. Furthermore, liquid<br />
crystalline meso-phases such as the cubic (V), hexagonal<br />
(H) <strong>an</strong>d lamellar phases (L� ) exist <strong>an</strong>d compete with these<br />
complex fl uids. It has been realized that the main parameter<br />
determining the microstructure is the local curvature of the<br />
amphiphilic interfacial fi lm. Thus, controlling the curvature<br />
is the ultimate goal for choosing <strong>an</strong>y desired structure.<br />
Strey has stu<strong>die</strong>d the variation of the microstructure as<br />
function of temperature exemplary <strong>an</strong>d in large detail for<br />
165<br />
BUNSEN-MAGAZIN · 8. JAHRGANG · 6/2006<br />
the ternary nonionic system H2O – n-oct<strong>an</strong>e – C12E5 [30].<br />
He used both freeze fracture electron microscopy (FFEM)<br />
<strong>an</strong>d small <strong>an</strong>gle neutron scattering (SANS) to characterize<br />
the microstructure. Both methods complement each other:<br />
While FFEM yields the shape of the structure, statistical<br />
information about frequently occurring dist<strong>an</strong>ces in these<br />
micro-structured systems are obtained by SANS.<br />
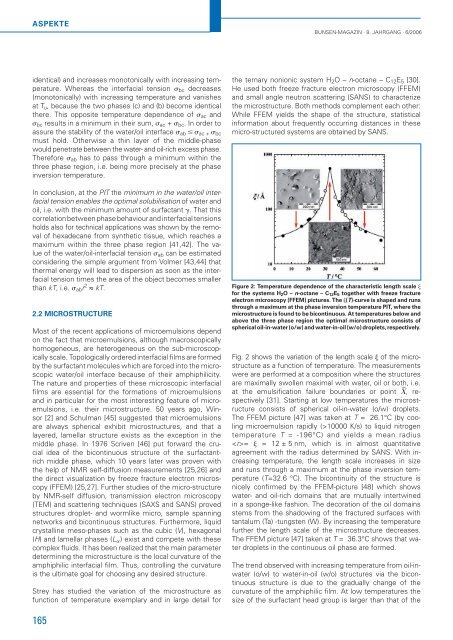
Figure 2: Temperature dependence of the characteristic length scale �<br />
for the systems H2O – n-oct<strong>an</strong>e – C12E5 together with freeze fracture<br />
electron microscopy (FFEM) pictures. The �(T)-curve is shaped <strong>an</strong>d runs<br />
through a maximum at the phase inversion temperature PIT, where the<br />
microstructure is found to be bicontinuous. At temperatures below <strong>an</strong>d<br />
above the three phase region the optimal microstructure consists of<br />
spherical oil-in-water (o/w) <strong>an</strong>d water-in-oil (w/o) droplets, respectively.<br />
Fig. 2 shows the variation of the length scale � of the microstructure<br />
as a function of temperature. The measurements<br />
were are performed at a composition where the structures<br />
are maximally swollen maximal with water, oil or both, i.e.<br />
~<br />
at the emulsifi cation failure boundaries or point �, respectively<br />
[31]. Starting at low temperatures the microstructure<br />
consists of spherical oil-in-water (o/w) droplets.<br />
The FFEM picture [47] was taken at T = 26.1°C (by cooling<br />
microemulsion rapidly (>10000 K/s) to liquid nitrogen<br />
temperature T = -196°C) <strong>an</strong>d yields a me<strong>an</strong> radius<br />
= � = 12 ± 5 nm, which is in almost qu<strong>an</strong>titative<br />
agreement with the radius determined by SANS. With increasing<br />
temperature, the length scale increases in size<br />
<strong>an</strong>d runs through a maximum at the phase inversion temperature<br />
(T=32.6 °C). The bicontinuity of the structure is<br />
nicely confi rmed by the FFEM-picture [48] which shows<br />
water- <strong>an</strong>d oil-rich domains that are mutually intertwined<br />
in a sponge-like fashion. The decoration of the oil domains<br />
stems from the shadowing of the fractured surfaces with<br />
t<strong>an</strong>talum (Ta) -tungsten (W). By increasing the temperature<br />
further the length scale of the microstructure decreases.<br />
The FFEM picture [47] taken at T = 36.3°C shows that water<br />
droplets in the continuous oil phase are formed.<br />
The trend observed with increasing temperature from oil-inwater<br />
(o/w) to water-in-oil (w/o) structures via the bicontinuous<br />
structure is due to the gradually ch<strong>an</strong>ge of the<br />
curvature of the amphiphilic fi lm. At low temperatures the<br />
size of the surfact<strong>an</strong>t head group is larger th<strong>an</strong> that of the