Evaluation of bactericidal and fungicidal activity of ferrocenyl or phenyl derivatives...
Ferrocene compounds
Ferrocene compounds
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Fe<br />
NH 2<br />
22 are inactive on h<strong>or</strong>mone-independent MDA-MB-231 breast<br />
cancer cells, while their <strong>ferrocenyl</strong> analogs 2, 3, 4 <strong>and</strong> 1 are amongst<br />
the most effective, with IC50 values between 0.5 mM <strong>and</strong> 0.8 mM.<br />
This difference is confirmed with compound 24, which is very<br />
active on MDA-MB-231 cancer cells, with an IC50 value <strong>of</strong> 0.09 mM,<br />
R<br />
2<br />
18<br />
Fe<br />
3<br />
R=Fc<br />
R=Ph<br />
OH<br />
OH<br />
2<br />
M. El Arbi et al. / Journal <strong>of</strong> Organometallic Chemistry 696 (2011) 1038e1048 1041<br />
OH<br />
OH<br />
NaH , THF<br />
1) EtONa, EtOH<br />
2)<br />
Br<br />
RCOCl<br />
Pyridine<br />
Fe<br />
NHCOR<br />
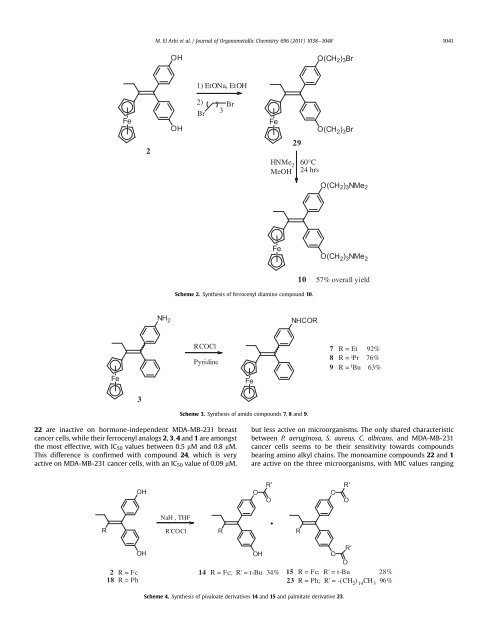
Scheme 3. Synthesis <strong>of</strong> amido compounds 7, 8 <strong>and</strong> 9.<br />
R'COCl R<br />
R<br />
14<br />
Br<br />
3<br />
7 R=Et 92%<br />
8 R= iPr 76%<br />
9 R= t Bu 63%<br />
but less active on micro<strong>or</strong>ganisms. The only shared characteristic<br />
between P. aeruginosa, S. aureus, C. albicans. <strong>and</strong> MDA-MB-231<br />
cancer cells seems to be their sensitivity towards compounds<br />
bearing amino alkyl chains. The monoamine compounds 22 <strong>and</strong> 1<br />
are active on the three micro<strong>or</strong>ganisms, with MIC values ranging<br />
O<br />
OH<br />
R'<br />
O<br />
R=Fc; R'=t-Bu 34%<br />
O<br />
R'<br />
R'<br />
O<br />
15<br />
O<br />
R=Fc; R'=t-Bu 28%<br />
23 R=Ph; R'=-(CH2 ) 14CH3 96%<br />
Scheme 4. Synthesis <strong>of</strong> pivaloate <strong>derivatives</strong> 14 <strong>and</strong> 15 <strong>and</strong> palmitate derivative 23.<br />
Fe<br />
HNMe 2<br />
MeOH<br />
Fe<br />
29<br />
60°C<br />
24 hrs<br />
10<br />
Scheme 2. Synthesis <strong>of</strong> <strong>ferrocenyl</strong> diamino compound 10.<br />
O(CH2)3Br<br />
O(CH 2) 3Br<br />
O(CH2)3NMe2<br />
O(CH 2 ) 3 NMe 2<br />
57% overall yield<br />
O