- Page 2:
Walter R. Johnson Atomic Structure
- Page 6:
Professor Dr. Walter R. Johnson Uni
- Page 10:
Preface This is a set of lecture no
- Page 14:
Contents 1 Angular Momentum .......
- Page 18:
Contents XI 6 Radiative Transitions
- Page 22:
1 Angular Momentum Understanding th
- Page 26:
1.1 Orbital Angular Momentum - Sphe
- Page 30:
1.1 Orbital Angular Momentum - Sphe
- Page 34:
Θl,m(θ) = (−1)l 2 l l! 1.1 Orbi
- Page 38:
1.2 Spin Angular Momentum 9 The Pau
- Page 42:
The matrix s 2 = s 2 x + s 2 y + s
- Page 46:
1.3 Clebsch-Gordan Coefficients 13
- Page 50:
1.3 Clebsch-Gordan Coefficients 15
- Page 54:
1.3 Clebsch-Gordan Coefficients 17
- Page 58:
1.4 Graphical Representation - Basi
- Page 62:
1.4 Graphical Representation - Basi
- Page 66:
1.5 Spinor and Vector Spherical Har
- Page 70:
aJLM = µν 1.5 Spinor and Vector
- Page 74: 1.5 Spinor and Vector Spherical Har
- Page 78: 2 Central-Field Schrödinger Equati
- Page 82: 2.2 Coulomb Wave Functions 2.2 Coul
- Page 86: 2.2 Coulomb Wave Functions 33 Pnℓ
- Page 90: and for σ = −(s +1)≤−1, J (
- Page 94: 2.3.1 Adams Method (adams) 2.3 Nume
- Page 98: 2.3 Numerical Solution to the Radia
- Page 102: 2.3 Numerical Solution to the Radia
- Page 106: 2.3 Numerical Solution to the Radia
- Page 110: 2.3 Numerical Solution to the Radia
- Page 114: 2.4 Quadrature Rules (rint) 47 wher
- Page 118: 2.5 Potential Models 49 with ζ = Z
- Page 122: 2.5 Potential Models 51 examining t
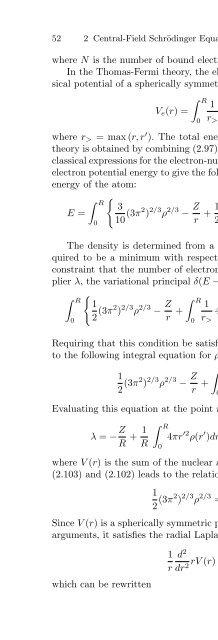
- Page 128: 54 2 Central-Field Schrödinger Equ
- Page 132: 56 2 Central-Field Schrödinger Equ
- Page 136: 58 2 Central-Field Schrödinger Equ
- Page 140: 60 2 Central-Field Schrödinger Equ
- Page 144: 62 2 Central-Field Schrödinger Equ
- Page 148: 64 2 Central-Field Schrödinger Equ
- Page 152: 66 2 Central-Field Schrödinger Equ
- Page 156: 68 2 Central-Field Schrödinger Equ
- Page 160: 70 2 Central-Field Schrödinger Equ
- Page 164: 72 3 Self-Consistent Fields The two
- Page 168: 74 3 Self-Consistent Fields v0(1s,
- Page 172: 76 3 Self-Consistent Fields − 1 2
- Page 176:
78 3 Self-Consistent Fields Ψ(r1,
- Page 180:
80 3 Self-Consistent Fields Rule 6
- Page 184:
82 3 Self-Consistent Fields I(nala)
- Page 188:
84 3 Self-Consistent Fields gabba
- Page 192:
86 3 Self-Consistent Fields The off
- Page 196:
88 3 Self-Consistent Fields Thus, w
- Page 200:
90 3 Self-Consistent Fields gives
- Page 204:
92 3 Self-Consistent Fields Normali
- Page 208:
4 2 24 16 8 0 94 3 Self-Consistent
- Page 212:
96 3 Self-Consistent Fields Eab··
- Page 216:
98 3 Self-Consistent Fields As in t
- Page 220:
100 3 Self-Consistent Fields where
- Page 224:
102 3 Self-Consistent Fields The (r
- Page 228:
104 3 Self-Consistent Fields of rad
- Page 232:
4 Atomic Multiplets In this chapter
- Page 236:
4.1 Second-Quantization 109 |ab ·
- Page 240:
Schrödinger Hamiltonian: 4.2 6-j S
- Page 244:
j1 j2 J12 j3 J J23 The quantity j
- Page 248:
4.3 Two-Electron Atoms 115 states c
- Page 252:
4.3 Two-Electron Atoms 117 E (1) 1s
- Page 256:
4.4 Atoms with One or Two Valence E
- Page 260:
4.4 Atoms with One or Two Valence E
- Page 264:
R = 4.5 Particle-Hole Excited State
- Page 268:
4.5 Particle-Hole Excited States 12
- Page 272:
4.6 9-j Symbols 127 charge where re
- Page 276:
4.7 Relativity and Fine Structure 1
- Page 280:
4.7 Relativity and Fine Structure 1
- Page 284:
E (1) J = vw,xy 4.7 Relativity and
- Page 288:
4.7 Relativity and Fine Structure 1
- Page 292:
138 5 Hyperfine Interaction & Isoto
- Page 296:
140 5 Hyperfine Interaction & Isoto
- Page 300:
142 5 Hyperfine Interaction & Isoto
- Page 304:
144 5 Hyperfine Interaction & Isoto
- Page 308:
146 5 Hyperfine Interaction & Isoto
- Page 312:
148 5 Hyperfine Interaction & Isoto
- Page 316:
150 5 Hyperfine Interaction & Isoto
- Page 320:
152 5 Hyperfine Interaction & Isoto
- Page 324:
154 5 Hyperfine Interaction & Isoto
- Page 328:
156 5 Hyperfine Interaction & Isoto
- Page 332:
158 6 Radiative Transitions E(r,t)=
- Page 336:
160 6 Radiative Transitions space o
- Page 340:
162 6 Radiative Transitions From (6
- Page 344:
164 6 Radiative Transitions U(t, t0
- Page 348:
166 6 Radiative Transitions d 2 wfi
- Page 352:
168 6 Radiative Transitions Substit
- Page 356:
170 6 Radiative Transitions to the
- Page 360:
172 6 Radiative Transitions ∞ 0
- Page 364:
174 6 Radiative Transitions Table 6
- Page 368:
176 6 Radiative Transitions 2zpxɛ
- Page 372:
178 6 Radiative Transitions As a sp
- Page 376:
180 6 Radiative Transitions Here, w
- Page 380:
182 6 Radiative Transitions 6.2.8 N
- Page 384:
184 6 Radiative Transitions Let us
- Page 388:
186 6 Radiative Transitions Oscilla
- Page 392:
188 6 Radiative Transitions where n
- Page 396:
190 6 Radiative Transitions The res
- Page 400:
192 6 Radiative Transitions Problem
- Page 404:
7 Introduction to MBPT In this chap
- Page 408:
7.1 Closed-Shell Atoms 7.1 Closed-S
- Page 412:
Closed-Shell: Third-Order Energy 7.
- Page 416:
Again, we write gmnab = where in t
- Page 420:
7.2 B-Spline Basis Sets 7.2 B-Splin
- Page 424:
The matrices A and B are given by A
- Page 428:
7.2 B-Spline Basis Sets 207 If we l
- Page 432:
7.3 Atoms with One Valence Electron
- Page 436:
7.3.2 Angular Momentum Decompositio
- Page 440:
7.3 Atoms with One Valence Electron
- Page 444:
7.3 Atoms with One Valence Electron
- Page 448:
7.4 Relativistic Calculations 217 s
- Page 452:
The radial matrix elements ML(ijkl)
- Page 456:
7.4 Relativistic Calculations 221 o
- Page 460:
7.4 Relativistic Calculations 223 2
- Page 464:
where ηkl is a symmetry factor def
- Page 468:
7.6 MBPT for Divalent Atoms and Ion
- Page 472:
7.6 MBPT for Divalent Atoms and Ion
- Page 476:
7.7 Second-Order Perturbation Theor
- Page 480:
7.7 Second-Order Perturbation Theor
- Page 484:
(b) 7.2. Prove: (a) (b) 0c mambmmm
- Page 488:
8 MBPT for Matrix Elements In Chapt
- Page 492:
8.1 Second-Order Corrections 239 Ta
- Page 496:
t RPA am = tam + bn t RPA ma = tma
- Page 500:
8.2 Random-Phase Approximation 243
- Page 504:
8.3 Third-Order Matrix Elements 245
- Page 508:
8.4 Matrix Elements of Two-Particle
- Page 512:
Energy (a.u.) 10 1 10 0 10 -1 10 -2
- Page 516:
8.5 CI Calculations for Two-Electro
- Page 520:
8.6 Second-Order Matrix Elements in
- Page 524:
8.6 Second-Order Matrix Elements in
- Page 528:
T (2) deriv (−1)J [JI][JF ] v≤
- Page 532:
8.7 Summary Remarks 259 8.2. Consid
- Page 536:
262 Solutions From the above, it fo
- Page 540:
264 Solutions l = 4 t := Table[(-1)
- Page 544:
266 Solutions This, in turn, can be
- Page 548:
268 Solutions top := top*(a+k-1); b
- Page 552:
270 Solutions P[n_, l_, r_] = Sqrt[
- Page 556:
272 Solutions Energies for Na from
- Page 560:
274 Solutions One finds [H, rk] =[c
- Page 564:
276 Solutions The S = 1 eigenstates
- Page 568:
278 Solutions (3s1/2 3s1/2) → [0]
- Page 572:
280 Solutions Thesumsoverµ’s ass
- Page 576:
282 Solutions 〈F |VI|I〉 = 1 gi
- Page 580:
284 Solutions δν =5× 3 4 × 0.91
- Page 584:
286 Solutions 5.5 Normal Mass Shift
- Page 588:
288 Solutions Extracting the coeffi
- Page 592:
290 Solutions 6.3 The Al ground sta
- Page 596:
292 Solutions 6.4 Heliumlike B: (a)
- Page 600:
294 Solutions (d) The 5d 3/2 state
- Page 604:
296 Solutions (c) He (1s2s) 3 S1- T
- Page 608:
298 Solutions where, χ (1) ma =
- Page 612:
300 Solutions Problems of Chapter 8
- Page 616:
302 Solutions i∆T (2) wv = χam
- Page 620:
304 References [17] A. R. Edmonds.
- Page 624:
Index LS coupled states first-order
- Page 628:
graphical rules 3j symbols, 20 arro
- Page 632:
second quantization, 107 second-ord