You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
ISSN 1862-2879<br />
Issue 1/2011 Vol. 7<br />
<strong>EDI</strong> Journal<br />
European Journal for<br />
Dental Implantologists<br />
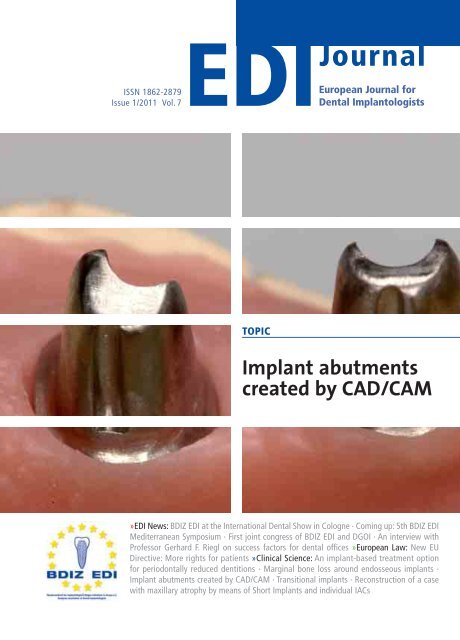
TOPIC<br />
Implant abutments<br />
created by CAD/CAM<br />
»<strong>EDI</strong> News: BDIZ <strong>EDI</strong> at the International Dental Show in Cologne · Coming up: 5th BDIZ <strong>EDI</strong><br />
Mediterranean Symposium · First joint congress of BDIZ <strong>EDI</strong> and DGOI · An interview with<br />
Professor Gerhard F. Riegl on success factors for dental offices »European Law: New EU<br />
Directive: More rights for patients »Clinical Science: An implant-based treatment option<br />
for periodontally reduced dentitions · Marginal bone loss around endosseous implants ·<br />
Implant abutments created by CAD/CAM · Transitional implants · Reconstruction of a case<br />
with maxillary atrophy by means of Short Implants and individual IACs
The Exacone implant is included in the libraries<br />
of the most common implant planning softwares<br />
(SimPlant® Materialise, Implant3D Medialab, Sicat-<br />
Galileos Sirona) to permit an accurate treatment<br />
plan and offer the possibility of fabricating surgical<br />
guides for a computer-assisted surgery.<br />
MultiTech is the new abutment specifi cally created<br />
for CAD/CAM technology used in restorative<br />
implantology. Its distinctive design favours its<br />
capturing through dental laboratory or intra-oral<br />
scanners and the customizing of its emergence<br />
profi le and angulation.<br />
Thanks to the absence of a screw hole and its<br />
consequently high mechanical strength, the geometry<br />
and specifi c surface of its bonding portion, MultiTech<br />
allows the realization of a fully patient-customized<br />
abutment. MultiTech offers the option to choose<br />
without any restriction among the several CAD/CAM<br />
methods using aesthetic materials like zirconium<br />
oxide and lithium disilicate.<br />
Orthodontics and Implantology<br />
LEONE S.p.a ��� �� � ���������� �� � ����� ����� ���������� ������� ������� � ����� ������������ ��� ������������� � ������������� � www.leone.it
The 34rd International Dental Show (IDS) in Cologne will again<br />
offer many surprises in terms of products and procedures. We<br />
are anxious to learn whether one of the major implant manufacturers<br />
will dare to embark on the zirconia implant “adventure”<br />
at IDS. Manufacturers have long been announcing<br />
ceramic implants, but they have yet to present them in the<br />
form of products ready for the market. Their presumed excellent<br />
biocompatibility and aesthetic shade would make these<br />
implants highly desirable for use in aesthetic dentistry, provided<br />
they can withstand continuous functional loads and feature<br />
abutments that are prefabricated but customizable.<br />
Innovative implant surfaces and treatment concepts based<br />
on angulated abutments definitely deserve mention. Without<br />
constant innovation and ongoing improvements to medicinal<br />
products, we would have fewer implant systems, implant surfaces<br />
and bone replacement materials today. As it is, however,<br />
implant dentistry has a large number of highly specific surgical<br />
techniques and materials available for a broad range of indications.<br />
But treatment providers and patients alike ask questions<br />
about the right choice of products and, especially, product safety.<br />
For example, the promotion of conditioned surfaces hydroxylated<br />
with hydroxide ions shortly before insertion to achieve<br />
better primary stability and improved osseointegration during<br />
the healing phase begs the question of whether reliable clinical<br />
studies are available to show that these specially conditioned<br />
surfaces actually result in shortened healing times.<br />
Another focus at IDS 2011 will be on biotechnological strategies<br />
for osteogenesis. We are facing an almost endless proliferation<br />
of synthetic bone replacement materials today. Some<br />
materials serve as placeholders for natural bone formation,<br />
while other types of materials are designed to replace autologous<br />
bone grafts. How well-proven and how successful are<br />
products such as porcine collagen matrix “straight from the<br />
blister pack”, designed to replace autologous connective-tissue<br />
grafts in covering recessions and augmented soft tissue?<br />
In addition to these developments in dental materials, new<br />
digital devices are coming more and more into focus with<br />
dental practitioners – in diagnostics and therapy alike.<br />
Products range from three-dimensional x-ray units and<br />
computer-guided implant placement all the way to optical<br />
scanners replacing conventional impression-taking and even<br />
automated dental technology procedures.<br />
In terms of diagnostics, many companies now offer software<br />
for computer-assisted treatment planning based on CBCT<br />
images. Oral implantologists will take the opportunity to scan<br />
IDS for software solutions capable of processing data for as<br />
many popular implant systems as possible.<br />
CAD/CAM procedures for manufacturing implant-supported<br />
restorations include opto-digital processes that dispense with<br />
plaster casts altogether. Single-session chairside restorations<br />
using scanner technology could be unveiled at IDS. Automated<br />
processes in dental technology are supposed to reduce cost.<br />
The industry has promised that custom components, e.g. for<br />
the aesthetic zone – which now require manual fabrication –<br />
could be produced in this way.<br />
Doubtlessly, recent innovation within oral implantology has<br />
been driven in large part by scientific progress and by the products<br />
developed by the dental industry. Prompted by an everincreasing<br />
demand by dentists and their patients, new products,<br />
new processes and improved therapeutic methods have<br />
been brought to market for many indications – from new<br />
approaches to bone augmentation and novel procedures in<br />
laser technology to newer materials, not least the now already<br />
familiar zirconia. Coming from a high level of achievement and<br />
extremely high success rates compared with other medical procedures,<br />
ever better results and shorter treatment times are<br />
not easy to attain. There are limits to what nature will allow us<br />
to do. In the light of this realization, it is all the more important<br />
for oral implantologists to avail themselves of opportunities<br />
for continuing education – to keep up with technical innovations<br />
and new materials, for the benefit of the patients and<br />
their own.<br />
Sincerely,<br />
Christian Berger, Kempten/Germany<br />
President of BDIZ <strong>EDI</strong><br />
<strong>EDI</strong><br />
Editorial<br />
Training and<br />
education<br />
must keep up<br />
with progress<br />
3
4 <strong>EDI</strong><br />
Table of Content<br />
<strong>EDI</strong> News<br />
The dental world assembles in Cologne<br />
Kicking off IDS 2011 8<br />
CAD/CAM developments at breathtaking speed<br />
Interview with Professor Joachim E. Zöller 10<br />
Meeting Point Implantology 2011<br />
BDIZ <strong>EDI</strong> at the International Dental Show<br />
in Cologne 14<br />
Implantology guide 16<br />
Implantological challenges on Portugal’s coast<br />
5th BDIZ <strong>EDI</strong> Mediterranean Symposium,<br />
10–17 June 2011 20<br />
Implantology and the dental team<br />
15th BDIZ <strong>EDI</strong> Symposium · 8th International<br />
Annual Congress of the DGOI 24<br />
PrintStar 2010 nomination for BDIZ <strong>EDI</strong> konkret<br />
“Academy Award” of the German printing world 30<br />
BDIZ <strong>EDI</strong> Administrator, Bonn office<br />
Career notes: Dr Dirk U. Duddeck 31<br />
Biomaterials under scrutiny<br />
20th International Expert Symposium for<br />
Regenerative Methods in Medicine and Dentistry 32<br />
Haemophilia’s little sister<br />
Third anniversary of the von Willebrand<br />
syndrome (vWS) network 34<br />
Finding the right patients<br />
An interview with Professor Gerhard F. Riegl on<br />
success factors for dental offices 36<br />
Europe Ticker 40<br />
European Law<br />
New EU Directive: More rights for patients 44<br />
On the legality of prior authorization requirements<br />
for outpatient treatment requiring the use of<br />
major medical devices in a different member state 48<br />
Clinical Science<br />
A new therapeutic approach<br />
An implant-based treatment option for<br />
periodontally reduced dentitions 50<br />
Marginal bone loss around endosseous implants<br />
Comparison of the bone response to dental<br />
implants with threaded and non-threaded necks 54<br />
Roads to implant abutments<br />
Implant abutments created by CAD/CAM 64<br />
Clinical Science<br />
Transitional implants<br />
A new approach to ridge restoration in cases of<br />
severe horizontal resorption 78<br />
A perfect fit<br />
Reconstruction of a case with maxillary atrophy<br />
by means of Short Implants and individual IACs<br />
(Integrated Abutment Crowns) 86<br />
Business & Events<br />
Innovations by Dentsply Friadent at IDS 2011 94<br />
An interview with Frank Bartsch,<br />
Trade Marketing Manager at Carestream Health 96<br />
An interview with Professor Daniel Buser and<br />
Professor Mariano Sanz on the International<br />
Osteology Symposium in Cannes 98<br />
MIS Global Conference, Cancun, Mexico,<br />
19 to 21 May 2011 100<br />
Thommen Connecting Science Workshop<br />
during the International Osteology Symposium 101<br />
TV-Wartezimmer’s market share on the rise 102<br />
H&H Webranking survey: Nobel Biocare’s<br />
website breaks top 5 103<br />
News and Views<br />
Editorial 3<br />
Imprint 6<br />
Product Reports 104<br />
Product News 107<br />
Calendar of Events/Publishers Corner 122<br />
Page 82:<br />
Inserting a<br />
transitional<br />
implant<br />
with a<br />
square tip.
© MIS Corporation. All rights Reserved.<br />
Explore<br />
360° Implantology
6<br />
<strong>EDI</strong><br />
Imprint<br />
<strong>EDI</strong><br />
European Journal for Dental Implantologists<br />
a BDIZ <strong>EDI</strong> publication<br />
published by teamwork media GmbH, Fuchstal<br />
Association: The European Journal for Dental Implantologists (<strong>EDI</strong>)<br />
is published in cooperation with BDIZ <strong>EDI</strong>.<br />
Publisher Board<br />
Members:<br />
Christian Berger<br />
Professor Joachim E. Zöller<br />
Dr Detlef Hildebrand, Dr Thomas Ratajczak<br />
Editor in Chief: Ralf Suckert, r.suckert@teamwork-media.de<br />
Editors: Anita Wuttke, Phone: +49 89 72069-888, wuttke@bdizedi.org<br />
Simone Stark, Phone: +49 8243 9692-34, s.stark@teamwork-media.de<br />
Scientific Board: Professor Alberico Benedicenti, Genoa Dr Marco Degidi, Bologna<br />
Dr Eric van Dooren, Antwerp Professor Rolf Ewers, Vienna<br />
Professor Antonio Felino, Porto Dr Jens Fischer, Bern<br />
Dr Roland Glauser, Zurich Professor Ingrid Grunert, Innsbruck<br />
Dr Detlef Hildebrand, Berlin Dr Axel Kirsch, Filderstadt<br />
Professor Ulrich Lotzmann, Marburg Professor Edward Lynch, Belfast<br />
Dr Konrad Meyenberg, Zurich Professor Georg Nentwig, Frankfurt<br />
Dr Jörg Neugebauer, Landsberg Professor Hakan Özyuvaci, Istanbul<br />
Professor Georgios Romanos, Rochester Luc and Patrick Rutten, MDT, Tessenderlo<br />
Dr Henry and Maurice Salama, Atlanta Dr Ashok Sethi, London<br />
Ralf Suckert, Fuchstal Professor Joachim E. Zöller, Cologne<br />
All case reports and scientific documentations are peer reviewed by the international editorial board<br />
of “teamwork – Journal of Multidisciplinary Collaboration in Restorative Dentistry“.<br />
Project Management<br />
& Advertising:<br />
Marianne Steinbeck, MS Media Service, Badstraße 5, D-83714 Miesbach<br />
Phone: +49 8025 5785, Fax: +49 8025 5583, ms@msmedia.de, www.msmedia.de<br />
Publishers: teamwork media Verlags GmbH, Hauptstr. 1, D-86925 Fuchstal<br />
Phone: +49 8243 9692-11, Fax: +49 8243 9692-22<br />
service@teamwork-media.de, www.teamwork-media.de<br />
Layout: Sigrid Eisenlauer; teamwork media GmbH<br />
Printing: J. Gotteswinter GmbH; Munich<br />
Publication Dates: March, June, September, December<br />
Subscription Rates: Annual subscription: Germany € 40 including shipping and VAT. All other countries € 58 including shipping. Subscription<br />
payments must be made in advance. Ordering: in written form only to the publisher. Cancellation deadlines: in<br />
written form only, eight weeks prior to end of subscription year. Subscription is governed by German law. Past issues<br />
are available. Complaints regarding nonreceipt of issues will be accepted up to three months after date of publication.<br />
Current advertising rate list from 1/1/2011.<br />
ISSN 1862-2879<br />
Payments: to teamwork media GmbH;<br />
Raiffeisenbank Fuchstal BRC 733 698 54 Account No.100 416746<br />
Copyright and<br />
Publishing Rights:<br />
All rights reserved. The magazine and all articles and illustrations therein are protected by copyright. Any utilization<br />
without the prior consent of editor and publisher is inadmissible and liable to prosecution. No part of this publication<br />
may be produced or transmitted in any form or by any means, electronic or mechanical including by photocopy, recording,<br />
or information storage and retrieval system without permission in writing from the publisher. With acceptance of<br />
manuscripts the publisher has the right to publish, translate, permit reproduction, electronically store in databases, produce<br />
reprints, photocopies and microcopies. No responsibility shall be taken for unsolicited books and manuscripts. Articles<br />
bearing symbols other than of the editorial department or which are distinguished by the name of the authors represent<br />
the opinion of the afore-mentioned, and do not have to comply with the views of BDIZ <strong>EDI</strong> or teamwork media<br />
GmbH. Responsibility for such articles shall be borne by the author. All information, results etc. contained in this publication<br />
are produced by the authors with best intentions and are carefully checked by the authors and the publisher. All<br />
cases of liability arising from inaccurate or faulty information are excluded. Responsibility for advertisements and other<br />
specially labeled items shall not be borne by the editorial department.<br />
Copyright: teamwork media GmbH . Legal Venue: Munich
© MIS Corporation. All rights Reserved.<br />
Enjoy<br />
The Best Cancun Has To Offer...
8<br />
<strong>EDI</strong><br />
<strong>EDI</strong> News<br />
IDS will be held in Halls 2, 3, 4, 10 and 11 this year.<br />
As in 2009, BDIZ <strong>EDI</strong> can be found in Hall 11.2, Aisle O,<br />
Stand 059 – again jointly with the legal offices of<br />
Ratajczak & Partners.<br />
The organizers – the Society for the Promotion of the<br />
Dental Industry (GDFI), the commercial arm of the<br />
Association of German Dental Manufacturers (VDDI),<br />
in cooperation with Koelnmesse, Cologne – expect<br />
approximately 1,900 exhibitors from 56 countries,<br />
with international exhibitors taking a 65 per cent<br />
share. This year, IDS will additionally utilize Hall 2 of<br />
the Cologne fairgrounds, for a gross exhibition area<br />
of 143,000 square metres or about 20 football fields.<br />
“Given the tremendous response on the part of our<br />
exhibitors, we hope to repeat the performance of<br />
2009, when more than 100,000 visitors attended.<br />
This will further consolidate and expand the position<br />
of IDS as a global beacon of the dental industry, not<br />
only with regard to the number of exhibitors, floor<br />
space or international attendance, but also with<br />
regard to the number of visitors”, as the organizers<br />
said in a joint statement.<br />
In addition to Germany, the most well-represented<br />
countries include Italy, the U.S., South Korea and<br />
Switzerland. In addition, there has been sizable<br />
growth in terms of international cooperation stands<br />
under the auspices of public or private export-promoting<br />
organizations or associations. Fourteen such<br />
cooperations have been registered so far, from<br />
Argentina, Australia, Brazil, Bulgaria, China, Japan,<br />
Israel, Italy, Pakistan, Russia, South Korea, Spain, Taiwan<br />
and the U.S. For 185 exhibitors this will be the<br />
first time they are represented in Cologne.<br />
One well-established feature of the IDS program is<br />
Speaker’s Corner in Hall 3.1, right next to the south<br />
entrance, where IDS exhibitors will be presenting<br />
Kicking off IDS 2011<br />
The dental world<br />
assembles in<br />
Cologne<br />
What is new at IDS 2011? Visitors to the International Dental Show in<br />
Cologne are looking forward to finding out. On 22 March 2011, the<br />
gates will open for the world’s largest fair for dentistry and dental<br />
technology. The first day is reserved for professional visitors. From<br />
23 to 26 March, Halls 2, 3, 4, 10 and 11 will be open to the general public.<br />
This year’s focus will be on endodontics and oral implantology.<br />
information on new products, services and procedures<br />
throughout the fair. Expert speakers will report<br />
on recent results and breakthroughs in science and<br />
technology.<br />
To make the most of their time at IDS 2011, visitors<br />
are recommended to plan their visit ahead of time.<br />
For this purpose, IDS now offers a separate app for<br />
iPhones, Blackberries, Android and other mobile systems<br />
comprising the IDS catalogue and an innovative<br />
navigation system for mobile devices guiding visitors<br />
safely through the halls and to the stands they wish<br />
to see.<br />
Arrival<br />
Both your trip and your stay can be easily arranged<br />
online ahead of time, the same is true of registration<br />
and ticketing at the IDS online shop. The IDS e-ticket<br />
not only gives you access to the IDS but doubles as<br />
your ticket for local transportation. The official IDS<br />
2011 airline is Lufthansa, offering visitors departing<br />
from more than 250 international<br />
cities in 100 countries special airfare<br />
rates. You may also obtain<br />
reduced-price train tickets on<br />
Deutsche Bahn to take you to IDS.<br />
The www.hotelzimmerbuchung.com<br />
website offers reservation of facilities<br />
for hotel rooms in Cologne<br />
and vicinity. For more information,<br />
please visit the IDS 2011 website at<br />
www.ids-cologne.de AWU<br />
IDS at a glance<br />
Getting started p. 8<br />
In depth p. 10<br />
The BDIZ <strong>EDI</strong> stand p. 14<br />
Implantology guide p. 16<br />
Opening hours<br />
23 to 26 March 2011<br />
Visitors: 9 am to 6 pm daily
© MIS Corporation. All rights Reserved.<br />
Three Days of 360° Implantology. lantology. Explore 360°<br />
Implantology: A complete world ld of dental implant resources<br />
from MIS. An up to date range e of researches, technologies<br />
and knowledge. And... Enjoy oy the full spectrum of the<br />
luxurious Cancun fun.<br />
MIS offers a wide range of implants, innovative e kits and<br />
accessories that provide creative and simple solutions<br />
for the varied challenges of implant dentistry. y.<br />
Make it Simple<br />
®<br />
For further information please visit:<br />
www.mis-events.com/cancun Or visit us at the<br />
IDS - International Dental Show, Cologne 22-26.03.2011<br />
Hall 4 Level 2 Booth Number 10L/19N
10<br />
<strong>EDI</strong><br />
<strong>EDI</strong> News<br />
Interview with Professor Joachim E. Zöller<br />
CAD/CAM developments<br />
at breathtaking speed<br />
What expectations do implant dentists have of IDS 2011? What innovations make sense, and where would healthy scepticism<br />
be indicated? Professor Joachim E. Zöller, Vice President of BDIZ <strong>EDI</strong> and scientific director of its symposia, answered the<br />
editorial team’s questions on the future of oral implantology as seen from the perspectives of the user and the scientist.<br />
Professor Joachim E. Zöller<br />
Professor Zöller, what innovations do you expect to<br />
see at IDS 2011?<br />
I would think that true innovation can be expected<br />
in the area of CAD/CAM-supported fabrication of<br />
implant-supported dentures, and here especially in<br />
digital impressions. This is where development progresses<br />
rapidly, and we can soon expect these procedures<br />
to become a standard adjunct in state-of-theart<br />
dentistry. We will also see some new developments<br />
in implant surfaces and bone replacement<br />
materials, but these I would not go so far as to call<br />
true innovations. Furthermore, I believe that we will<br />
be seeing the beginnings of a restructuring of the<br />
implant market. The roster of exhibitors has changed<br />
significantly since 2009. A recently published analytical<br />
study by Morgan Stanley on the dental implant<br />
market in Germany is highly interesting in this context.<br />
This study re-evaluates the major players and<br />
points out the changes that are taking place.<br />
There have been rumours that one of the big players<br />
among implant manufacturers might present a zirconia<br />
implant. Would not this represent a breakthrough<br />
for ceramic implants?<br />
At this point, ceramic implants have less than a five<br />
per cent market share, so they only play a minor role.<br />
The main reason is that ceramic implants – especially<br />
in cantilever situations – still have disadvantages<br />
compared to titanium. A breakthrough could occur<br />
if we saw significant progress in surface treatments<br />
and if the stability of the connection between two<br />
segmented ceramic implants was similar to that<br />
between titanium implants.<br />
Speaking of implant surfaces: What is your take on<br />
surface conditioning, where surfaces are hydroxylated<br />
with hydroxide ions shortly before placement to<br />
achieve extra stability and better osseointegration<br />
during the healing phase?<br />
Healing rates in healthy patients exceed 95 per<br />
cent. In other words, current hydrophilic surfaces<br />
based on the acid-etching technology can be considered<br />
very mature products. Not least for statistical<br />
reasons, further optimization would be possible only<br />
if someone invented a completely new surface offering<br />
very significant extra benefits. The current “innovations”,<br />
in my opinion, do not fit this bill. Rather,<br />
what we hear is mostly the industry’s sales arguments.<br />
As far as the healing phase is concerned, we<br />
are actually very familiar with the processes governing<br />
physiological bone healing. Thinking that we can<br />
cheat nature is a mistaken belief.<br />
But are there any reliable studies showing that shorter<br />
healing times can be achieved only with specially<br />
conditioned surfaces?<br />
Consolidating bone fractures will be tentatively<br />
stable after six weeks and functionally stable after
Cone-beam<br />
computed<br />
tomography<br />
(CBCT) is making<br />
further advances.<br />
<strong>EDI</strong><br />
<strong>EDI</strong> News<br />
eight to ten weeks. If we allow for a safety margin of<br />
two weeks, we have arrived at today’s healing times.<br />
My question is: Who is actually interested in shortened<br />
healing times? Does shortening the healing<br />
times by two to four weeks really help the patient?<br />
Or is this a phenomenon similar to the immediateplacement<br />
fad? Here, too, we have seen that this only<br />
works reliably in certain situations.<br />
Can we expect anything revolutionary at IDS regarding<br />
abutments and superstructures? What are you<br />
personally on the lookout for?<br />
I think we will see the trend toward custom abutments<br />
continue. We have already seen a lot of developments<br />
in this field in recent years. But here, too,<br />
ceramic abutments will be accepted only in the aesthetic<br />
zone. We continue to experience massive problems<br />
with these abutments in the posterior region.<br />
CAD/CAM procedures for manufacturing implant-supported<br />
restorations now include opto-digital processes<br />
that dispense with plaster casts altogether. Automated<br />
processes are supposed to reduce cost. The industry<br />
has promised that custom components, e.g. for the<br />
aesthetic zone, which now require manual fabrication,<br />
could be produced in this way. Single-session<br />
chairside restorations using scanner technology could<br />
be unveiled at IDS. Does that not sound promising?<br />
The important breakthrough for CAD/CAM procedures<br />
for manufacturing implant-supported restorations<br />
will come once intraoral digital impressions are<br />
ready for use in clinical practice. Here I expect digital<br />
images to be available in a matter of milliseconds,<br />
just as with present-day impression trays. Once this<br />
problem has been solved, and solved in a practical<br />
manner, I am sure that this impression technique will<br />
replace all conventional laboratory procedures, with<br />
very few exceptions. This will not only mark the end<br />
of all outsourcing of laboratory services to far-away<br />
countries – domestic laboratories, too, will have to<br />
completely revamp their processes in order to survive.<br />
11<br />
����������������<br />
�����������<br />
�����������������������<br />
�������������������������<br />
�������������������������<br />
�������������<br />
����������������������<br />
������������������<br />
�������������<br />
�����������������<br />
����������������<br />
���������������������<br />
���<br />
���<br />
���<br />
���<br />
�<br />
���������<br />
��������<br />
��������������������������<br />
������������������������������������������<br />
��������������������������������������<br />
���������������������������������<br />
����������� ����<br />
���������������<br />
�������������������������������������<br />
��������������������������������������<br />
��������������
12<br />
<strong>EDI</strong><br />
<strong>EDI</strong> News<br />
Just imagine: In the future you can have a scan taken<br />
of your adult daughter, and if she really should lose<br />
a tooth later on, she will simply have one milled. I<br />
am certain that we will see new possibilities evolve<br />
with tremendous speed.<br />
In diagnostics, the three-dimensional planning system<br />
hype appears to be subsiding. Although CBCT units<br />
have significantly come down in price, they still cost<br />
twice as much as conventional radiography. What circumstances,<br />
in your opinion, would justify the higher<br />
cost, extra time and the extra training needed to evaluate<br />
the results?<br />
I am sure clinical reality will provide the answer for<br />
that. Whether we like it or not: implantological diagnostics<br />
will increasingly be three-dimensional, with<br />
two-dimensional procedures restricted to very clearcut<br />
clinical situations. So CBCT will definitely not be<br />
reserved for particularly severe or complicated cases,<br />
as we thought not too long ago; time has passed us<br />
by. And once we have precision diagnostics available<br />
anyway, we will want to transfer the treatment planning<br />
data to the result as well. Over the long haul,<br />
the patients themselves will expect this. I believe<br />
that navigated template-guided implant insertion<br />
will become standard in the foreseeable future, a<br />
development that will be driven not least by cheap<br />
CAD/CAM-produced templates.<br />
I am sure you have been looking particularly closely at<br />
biotech strategies for osteogenesis. There is an almost<br />
endless proliferation of synthetic bone replacement<br />
materials. How well-proven and how successful are<br />
products such as porcine collagen matrix “straight<br />
from the blister pack” designed to replace autologous<br />
connective-tissue grafts in covering recessions and<br />
augmented soft tissue?<br />
It is true that “new” bone replacement materials<br />
are marketed at a rapid pace, but many allegedly new<br />
products are simply old wine in new bottles. For<br />
many of these materials, there has been hardly any<br />
clinical testing, or at least not at a higher level of evidence.<br />
Here the industry benefits from a tendency on<br />
the part of many dentists to try something “new” in<br />
order to chase the potential benefit rather than opting<br />
for well-proven products.<br />
When do you think that bone cultivated from bonemarrow<br />
stem cells will be ready for the market?<br />
Billions are being invested in stem cell research<br />
every year, all over the world. This field of research<br />
receives the most support. Ultimately, I believe that<br />
we will be seeing entirely new ways of practicing<br />
medicine opening up. Take diabetes mellitus or<br />
myocardial infarction. Here we will be able to effec-<br />
tively treat the causes rather than<br />
prescribing drugs to treat the<br />
symptoms. Compared to that, producing<br />
bone for various medical<br />
applications will be a minor problem.<br />
We will be able to generate<br />
bone tissue, not only complete<br />
with cortical and spongious bone<br />
but also complete with all other<br />
components such as blood vessels.<br />
This so-called tissue printing is<br />
the objective of massive research<br />
efforts. But we will probably see<br />
another five to ten years go by<br />
before we can proceed to clinical<br />
testing of initial applications. Even<br />
though stem-cell products are<br />
introduced every now and then,<br />
we are still a long way from any<br />
mature clinically products.<br />
What does the future hold for<br />
bone augmentation? Could laser<br />
technology offer new impulses?<br />
Industry protagonists hope to<br />
develop consistently porous<br />
moulded bodies to replace autolo-<br />
Bone replacement materials:<br />
gous grafts. They could improve<br />
The familiar and the new.<br />
the success rates in lateral-access<br />
augmentation procedures using bone replacement<br />
materials. I do not believe that bony integration of<br />
these moulded bodies will be good enough in the<br />
case of vertical augmentation. Overall, these products<br />
might constitute an interim solution until viable<br />
stem-cell products are available.<br />
Our last question will prompt you to take a peek at<br />
the future: Can we expect to see new treatment concepts<br />
in oral implantology?<br />
I think that we will see new treatment concepts<br />
related to CAD/CAM-produced implant-supported<br />
restorations. Here we will see an avalanche of new<br />
technologies, with considerably falling costs as a<br />
consequence, making laboratory products significantly<br />
cheaper. With regard to the surgical aspects of<br />
oral implantology, we would be well advised to continue<br />
standardizing current treatment concepts and<br />
their variants so that patients can be offered low-risk<br />
treatment and high success rates. This level of quality,<br />
however, will always have to have its price.<br />
Professor Zöller, thank you very much for this insightful<br />
look into the future of oral implantology.<br />
AWU
© Nobel Biocare Services AG, 2011. All rights reserved. Nobel Biocare, the Nobel Biocare logotype and all other trademarks are, if nothing else is stated or is evident from the context<br />
in a certain case, trademarks of Nobel Biocare<br />
Dual-function prosthetic<br />
connection.<br />
Bone-condensing property.<br />
NobelActive TM<br />
A new direction for implants.<br />
Adjustable implant orientation<br />
for optimal final placement.<br />
Meet us<br />
in Hall 4,<br />
A090/091<br />
NobelActive equally satisfies<br />
surgical and restorative clinical<br />
goals. NobelActive thread design<br />
progressively condenses bone<br />
with each turn during insertion,<br />
which is designed to enhance<br />
initial stability. The sharp apex<br />
and cutting blades allow surgical<br />
clinicians to adjust implant orientation<br />
for optimal positioning of the<br />
prosthetic connection. Restorative<br />
clinicians benefit by a versatile and<br />
secure internal conical prosthetic<br />
connection with built-in platform<br />
shifting upon which they can<br />
produce excellent esthetic results.<br />
Based on customer feedback and<br />
market demands for NobelActive,<br />
the product assortment has been<br />
expanded – dental professionals<br />
Built-in platform shifting.<br />
High initial stability,<br />
even in compromised<br />
bone situations.<br />
will now enjoy even greater flexi -<br />
bility in prosthetic and implant<br />
selection.<br />
Nobel Biocare is the world leader<br />
in innovative and evidence-based<br />
dental solutions.<br />
For more information, visit our<br />
website<br />
www.nobelbiocare.com<br />
Disclaimer: Some products may not be regulatory cleared/released for sale in all markets. Please contact the local Nobel Biocare sales office for current product<br />
assortment and availability.<br />
NobelActive A4 IDS <strong>EDI</strong> JOURNAL.indd 1 11-02-10 16.20.08
14<br />
<strong>EDI</strong><br />
<strong>EDI</strong> News<br />
BDIZ <strong>EDI</strong> at the International Dental Show in Cologne<br />
Meeting Point Implantology 2011<br />
We offer high-profile continuing education and training. Our support for dentists when it comes to private-patient billing issues<br />
in Germany is second to none. Like a well-trained rescue dog, BDIZ <strong>EDI</strong> sniffs out what implantological clinics are missing in terms<br />
of education and legal and accounting advice. The main beneficiaries of these efforts are BDIZ <strong>EDI</strong>’s members, whom we would<br />
like to welcome to the BDIZ <strong>EDI</strong> stand at IDS 2011 in Cologne (Hall 11.2, Aisle O, Stand 059) to meet the people behind the scenes.<br />
At the 34th IDS, BDIZ <strong>EDI</strong> will have a joint stand with<br />
the legal offices of Ratajczak & Partners. As an expert in<br />
medical law, Dr Thomas Ratajczak has been a knowledgeable<br />
partner of the BDIZ <strong>EDI</strong> Board and members<br />
for many years and provided legal counsel. “Our<br />
Meeting Point Implantology is meant to assemble<br />
and focus all the available expertise and to demonstrate<br />
the support that BDIZ <strong>EDI</strong> can offer implant<br />
dentists”, says BDIZ <strong>EDI</strong> President Christian Berger.<br />
New: Implantological guideline<br />
“Implants without augmentation”<br />
In early March, the European Consensus Conference<br />
(EuCC) held a meeting in Cologne under the auspices<br />
of BDIZ <strong>EDI</strong>. The result was a new implantological<br />
guideline, the sixth to be issued so far. At IDS, the<br />
guideline, which addresses the issue of implants<br />
without augmentation, will be available fresh off the<br />
press. The other five guidelines published to date, on<br />
immediate loading (2006), ceramics (2007), periimplantitis<br />
(2008), 3-D imaging (2009) and complications<br />
(2010) can be downloaded from the BDIZ <strong>EDI</strong><br />
website at www.bdizedi.org (change to English,<br />
select Professionals, choose the desired guidelines<br />
from the menu on the left).<br />
New: Testing implant surface materials<br />
The second qualitative and quantitative elemental<br />
analysis of the surfaces of more than 40 different<br />
makes of sterile-wrapped implants, presumably one<br />
of the most comprehensive worldwide, was conducted<br />
by BDIZ <strong>EDI</strong> jointly with the University of Cologne<br />
in 2010. Its findings, made possible only by the use of<br />
the scanning electron microscope, included improperly<br />
milled threads, implants with remarkably high<br />
levels of sandblasting residue and even implants<br />
with systematic organic contaminants. Significant<br />
improvements to a number of implants had already<br />
BDIZ <strong>EDI</strong> will be at Hall 11.2, Aisle O, Stand 059.<br />
followed the first publications of the results by BDIZ<br />
<strong>EDI</strong>. In a comprehensive follow-up study, numerous<br />
implants by other manufacturers are currently being<br />
examined using the same study protocol. The objective<br />
is to obtain an overview of the surface characteristics<br />
of as many implants on the market as feasible.<br />
For the first time, this research will also include zirconia<br />
implants, mini-implants and intermediate structures<br />
in the SEM analyses. If you are curious to find<br />
out how “your” implant has fared in this study, just<br />
visit us at the BDIZ <strong>EDI</strong> stand.<br />
Learn more at the BDIZ <strong>EDI</strong> stand!<br />
Any questions? We will be happy to provide answers.<br />
Of course, the BDIZ <strong>EDI</strong> Board will be present on-site:<br />
Presidents Christian Berger and Professor Joachim E.<br />
Zöller, Treasurer Dr Heimo Mangelsdorf, Secretary<br />
General Dr Detlef Hildebrand, Managing Director and<br />
Secretary Dr Stefan Liepe, as well as the Administrator<br />
of the BDIZ <strong>EDI</strong> Bonn office, Dr Dirk U. Duddeck. We<br />
will be happy to try to answer any questions you may<br />
have – whether on BDIZ <strong>EDI</strong> symposia, on curricula or<br />
on material testing procedures.<br />
AWU
110301_WH_AD_ELCOMED_A4_AEN_IDS:Layout 1 01.03.11 12:20 Seite 1<br />
80 Ncm<br />
Powerful for surgery<br />
120 Years W&H.<br />
Help us support SOS Children’s Villages!<br />
The new Elcomed from W&H: logical and easy to use. Uncompromising<br />
in performance: with a torque of up to 80 Ncm on the rotating instrument,<br />
the surgical drive unit guarantees smooth usage, which can be completely<br />
documented at no further cost thanks to an inte grated USB interface.<br />
These are just three of the many advantages of the new W&H Elcomed.<br />
Captivation and precision
16<br />
<strong>EDI</strong><br />
<strong>EDI</strong> News<br />
Implantology guide<br />
Company Hall Aisle Stand<br />
3M Espe AG 4.2 G + J 090 + 099<br />
+ 091<br />
ACTEON Germany GmbH 10.2 N + O 060 + 069<br />
Advanced Technology Research ATR s.r.l. 11.2 R 051<br />
AESCULAP AG 10.1 C + D 020 + 029<br />
Alpha-Bio tec. 4.2 G 020<br />
ANTHOGYR 11.1 C + D 040 + 041<br />
Aseptico, Inc. 10.2 T 015<br />
Astra Tech AB 3.2 A + C<br />
C + E<br />
010 + 019<br />
AVINENT IMPLANT SYSTEM, S.L. 4.1 D 040<br />
B.T.I. Deutschland GmbH 3.2 E + F 020 + 029<br />
Bego Implant Systems 10.2 M + N 018 + 029<br />
020 + 029<br />
N + O 028 + 029<br />
Bien Air 10.1 H + J 050 + 059<br />
BioHorizons GmbH 4.1 A 039<br />
BIOMET 3i 4.2 J + G 039 + 030<br />
BIOTECK s.r.l. 11.1 J 017<br />
Bontempi Medizintechnik GmbH 4.1 C 100<br />
BPI Biologisch Physikalische Implantate<br />
GmbH & Co. KG<br />
4.2 K 040<br />
B-Productions GmbH 10.2 R 068<br />
bredent medical GmbH & Co. KG 11.1 B + C 010 + 019<br />
BTI Biotechnology Institute, S.L. 3.2 E + F 020 + 029<br />
BTLock s.r.l. 11.1 F 038<br />
C. Hafner GmbH + Co. KG Goldund<br />
Silberscheideanstalt<br />
CAMLOG Biotechnologies AG 11.3 A + B<br />
+ C<br />
10.2 R 011<br />
010 + 019<br />
018 + 019<br />
Carestream 10.2 T + U 040 + 041<br />
+ 043<br />
Carl Martin GmbH 10.2 N + O 020 + 021<br />
Carlo de Giorgi SRL. 11.2 L 049<br />
Champions Implants 11.1 B 008<br />
Clinical House Europe GmbH 2.2 D + E 010 + 011<br />
Curasan 4.2 L + M 038 + 039<br />
Degradable Solutions 10.1 J 064<br />
DENTATUS AB 10.1 K 050<br />
DENTAURUM IMPLANTS GmbH 10.1 F 014<br />
DENTECH CORPORATION 10.2 V 023<br />
Dentech Dental Instruments<br />
Manufacturer & Trade<br />
4.2 G 008<br />
Dentegris Deutschland GmbH 11.2 K 051<br />
Dentium USA 4.2 K + L 060 + 061<br />
DENTSPLY Friadent GmbH 11.2 K + L 018 + 019<br />
020 + 021<br />
018 + 019<br />
020 + 021<br />
Company Hall Aisle Stand<br />
DEPPELER S.A. 10.2 T 025<br />
DIO Corporation 4.1 A 030<br />
DOT GmbH 11.2 N 063<br />
Dr. Ihde Dental GmbH 10.2 O 059<br />
Dyna Dental Engineering b.v. 10.2 S + T 068 + 069<br />
DZR 3.2 A 015<br />
EQUINOX M<strong>EDI</strong>CAL TECHNOLOGIES BV 10.1 B 048<br />
FairImplant 4.1 F 101<br />
Gebrüder Martin GmbH & Co. KG 4.2 G 028<br />
Geistlich 4.2 G 031<br />
General Implants GmbH 11.1 G 011<br />
Ghimas S.p.A. 4.1 B + C 070 + 071<br />
GlaxoSmithKline 11.3 K + L 020 + 029<br />
HADER SA 10.1 G + H 048 + 049<br />
Hager & Meisinger GmbH 10.1 G + H 019 + 028<br />
+ 029 +<br />
030 + 039<br />
Hager & Werken 4.1 A + B 070 + 079<br />
Hauschild & Co. KG 10.2 S + T 068 + 069<br />
Helmut Zepf Medizintechnik GmbH 10.1 C 041<br />
Henry Schein 10.2 L + M 040 + 049<br />
+ 048<br />
Heraeus Kulzer GmbH & Co KG 10.1 A + B<br />
+ C<br />
010 + 019<br />
010 + 019<br />
HI-TEC IMPLANTS LTD 3.2 F + G 028 + 029<br />
Hu-Friedy Mfg. Co., Inc. 10.1 D + E 040 + 041<br />
IDI SYSTEM (IMPLANTS DIFFUSION<br />
INTERNATIONAL)<br />
4.1 D 041<br />
Implant Direct 4.1 C 012<br />
Imtegra OHG 4.1 C 018<br />
Institut Straumann AG 4.2 G + K 080 + 089<br />
Intra-Lock International, Inc. 4.2 N 048<br />
J+K Chirurgische Instrumente GmbH 10.1 K 061<br />
Jakobi Dental Instruments 11.3 F 060<br />
KaVo 10.1 J + K<br />
H + J<br />
J + K<br />
J + K<br />
H + J<br />
Keystone 11.1 A 050<br />
K.S.I.-Bauer-Schraube GmbH 10.2 S 048<br />
Karl Hammacher GmbH 10.1 C 031<br />
KOHLER Medizintechnik GmbH & Co. KG 10.2 L 029<br />
030 + 031<br />
040 + 041<br />
010 + 019 +<br />
020 + 021<br />
028 + 029<br />
018 + 019<br />
Komet Gebr. Brasseler GmbH & Co. KG 10.2 U + V 010 + 019<br />
LEADER ITALIA SRL 10.2 U 019<br />
LEONE s.p.a. 4.1 A 068
The<br />
nnew<br />
e ew<br />
kkit<br />
it<br />
fo for or<br />
s ssuccess.<br />
uccess.<br />
Geistlich Combi-Kit Collagen –<br />
the best kit for successful<br />
and predictable results in<br />
ridge preservation and minor<br />
augmentations.<br />
Geistlich<br />
Bio-Oss ® Collagen and<br />
Geistlich Bio-Gide ®<br />
NEW<br />
combined in Geistlich<br />
Combi-Kit Collagen<br />
www.geistlich-pharma.com<br />
Visit us at the<br />
IDS in Cologne,<br />
Germany<br />
��March 22–26, 2011<br />
�����������<br />
���������������<br />
LEADING RE REGENERATION<br />
GGENERATION
18<br />
<strong>EDI</strong><br />
<strong>EDI</strong> News<br />
Company Hall Aisle Stand<br />
MATERIALISE DENTAL NV 4.2 J 019<br />
MECTRON S.P.A. 10.2 O + P 040 + 041<br />
med3D GmbH Implantology 10.2 R 011<br />
medentis medical GmbH 3.2 C + E 030 + 039<br />
Medizintechnik Gulden 11.3 B 014<br />
Medical Instinct 3.2 E + F 058 + 059<br />
MegaGen Implant Co., Ltd. 3.1 H 051<br />
Merz Dental GmbH 10.2 T + U 038 + 039<br />
Metoxit AG 4.1 B 006<br />
MIS 4.2 N + L 019 + 010<br />
MK1 Dental-Attachment GmbH 11.1 A 057<br />
J. Morita 10.2 R + S 040 + 049<br />
+ 042<br />
MOZO-GRAU, S.L. 4.1 D 078<br />
Nemris GmbH & Co. KG 4.1 B 029<br />
NEOSS GmbH 4.2 K + L 090 + 099<br />
Nobel Biocare Deutschland GmbH 10.1 D + E 010 + 019<br />
NOUVAG AG 11.1 F 059<br />
OMNIA S.p.A. 4.1 C + D 088 + 089<br />
Orangedental 11.2 M + N 040 + 049<br />
OSSTELL AB 3.2 E 070<br />
OSSTEM IMPLANT Co., Ltd. 4.1 A + B 010 + 019<br />
OT medical 3.2 F + G 030 + 039<br />
Otto Leibinger GmbH 4.2 G 100<br />
POLYDENTIA SA 10.2 R 050<br />
Promedia 10.2 V 029<br />
RESISTA Ing. Carlo Alberto Issoglio<br />
& C. S.R.L.<br />
10.2 T 049<br />
Resorba 3.2 A 048<br />
Riemser 3.2 C 040<br />
SAE DENTAL VERTRIEBS GmbH -<br />
INTERNATIONAL<br />
10.2 V 058<br />
Schlumbohm GmbH & Co. KG 10.2 U 030<br />
Schütz-Dental GmbH 10.1 G + H 010 + 019<br />
SICAT 10.2 O + P 010 + 029<br />
SIC invent AG 4.2 N + L 089 + 080<br />
Sirona Dental Systems GmbH 10.2 N + O<br />
+ P<br />
Si-tec GmbH 4.1 B 020<br />
Southern Implants GmbH 3.2 A 008<br />
steco-system-technik GmbH & Co. KG 11.1 D 008<br />
Stoma Dentalsysteme GmbH & Co. KG 10.2 U 011<br />
010 + 019<br />
+ 029 + 009<br />
Straumann GmbH 4.2 G + K 080 + 089<br />
Sybron Implant Solutions GmbH 10.1 J 029<br />
Talladium International 10.2 T 037<br />
T.B.R. GROUP SUDIMPLANT SA 11.2 N + O 030 + 031<br />
Tecnoss Dental s.r.l. 10.1 A 031<br />
Thommen Medical AG 4.2 N + L 091 + 090<br />
Company Hall Aisle Stand<br />
TRI dental 11.1 G 011<br />
Trinon Titanium GmbH 11.3 G 038<br />
Ustomed Instrumente Ulrich Storz<br />
GmbH & Co. KG<br />
10.1 A + B 048 + 049<br />
Wegold Edelmetalle AG 4.1 B + D 020 + 030<br />
+ 039<br />
Wieland Dental + Technik GmbH<br />
& Co. KG<br />
10.1 F + G 018 + 020<br />
+ 029<br />
W & H 10.1 C + D 018 + 019<br />
YDM CORPORATION 4.1 D + C 071 + 070<br />
ziterion GmbH 4.2 G 040<br />
ZL Microdent-Attachment GmbH<br />
& Co. KG<br />
10.1 P + Q 038 + 039<br />
Z-Systems AG 4.1 E 013<br />
Zimmer Dental 3.2 C + E 020 + 029<br />
Important addresses<br />
Company Hall Aisle Stand<br />
BDIZ <strong>EDI</strong> 11.2 O 059<br />
BZÄK/DGZMK/KZBV 11.2 O + P 050 + 059<br />
DAISY – Akademie und Verlag 11.2 S 009<br />
Deutscher Ärzte-Verlag 11.1 E + F 008 + 009<br />
DGOI 2.2 A 011a + 011<br />
DGZI 4.1 C 063<br />
Quintessenz Verlags-GmbH 11.2 N + O<br />
+ P<br />
008 + 009<br />
Spitta Verlag 11.2 P + Q 020/029<br />
teamwork media Verlag 11.1 F 008<br />
Zahnärztlicher Fachverlag/DZW 11.2 N + O 049 + 049
Zimmer Dental is evolving implant dentistry with the introduction<br />
of Trabecular Metal Material. With over a decade of clinical use in<br />
orthopaedics, Trabecular Metal Material’s highly biocompatible<br />
tantalum and 3D porous cellular structure have been clinically proven<br />
to create the potential for clinical ingrowth, or osseoincorporation. 1-3<br />
Bone’s ability to not only grow into the pores and around the struts,<br />
but also to interconnect, is what makes Trabecular Metal Material<br />
The Best Thing Next To Bone.<br />
To learn more, talk to a Zimmer sales representative or visit<br />
http://TrabecularMetal.zimmerdental.com.<br />
1. Bobyn JD, Poggie RA, Krygier JJ, Lewallen DF, Hanssen AD, Lewis RJ, Unger AS, O’Keefe TJ, Christie MH, Nasser S, Wood JE,<br />
Stulberg SD, Tanzer M. Clinical validation of a structural porous tantalum biomaterial for adult reconstruction. J Bone Joint<br />
Surg. 2004;86-A(Suppl 2):123-129. 2. Black J. Biological performance of tantalum. Clin Mater. 1994;16:167-173. 3. Bobyn JD.<br />
UHMWPE: the good, bad, & ugly. Fixation and bearing surfaces for the next millennium. Orthop. 1999;22(9):810-812.<br />
The Best Thing Next To Bone. <br />
Introducing Trabecular Metal TM<br />
Material to implant dentistry.<br />
Trabecular Metal Material<br />
Osseoincorporation<br />
The process of ingrowth<br />
Artistic Rendering<br />
www.zimmerdental.com<br />
©2010 Zimmer Dental Inc.<br />
Trabecular bone<br />
���������������������������������� ����������������
20<br />
<strong>EDI</strong><br />
<strong>EDI</strong> News<br />
5th BDIZ <strong>EDI</strong> Mediterranean Symposium, 10–17 June 2011<br />
Implantological challenges<br />
on Portugal’s coast<br />
In 2011, as in previous years, BDIZ <strong>EDI</strong> will continue its proven concept of holding continuing education<br />
courses outside Germany. This year’s Mediterranean Symposium will be held in Portugal.<br />
If you are looking for a high-class continuing education event and a relaxing environment for<br />
the whole family, the 5th BDIZ <strong>EDI</strong> Mediterranean Symposium will be the right choice for you.<br />
Together with its Portuguese partner organization,<br />
the Sociedade Portuguesa de Cirurgia Oral (SPCO),<br />
BDIZ <strong>EDI</strong> will be holding an international congress<br />
at Lisbon, the Portuguese capital, followed<br />
by a week of lectures and workshops – and quality<br />
time for you to unwind together with your family<br />
– in the Atlantic resort of Estoril, next to larger<br />
Cascais.<br />
The all-day symposium will take place at the<br />
Pestana Hotel in Lisbon on Saturday, 11 June 2011.<br />
The topic of the 5th Mediterranean Symposium<br />
will be ”Clinical Challenges in Oral Implantology.”<br />
Simultaneous interpretation will be available for<br />
the presentations of the Portuguese speakers; the<br />
German speakers will present in English. The scientific<br />
program has been compiled jointly by Professor<br />
Jo achim E. Zöller, Cologne, and Professor Antonio<br />
Felino, Porto.<br />
The venue for the continuing-education week will<br />
be the Atlantic Coast, a place of spectacular beauty.<br />
Cascais/Estoril offers sunshine, beach life and an<br />
attractive coastscape, sailing and golfing opportunities<br />
– and the area also has a lot to offer for those<br />
interested in history. Many buildings go back to the<br />
heydays of the Portuguese seafarers. Once again<br />
this year, symposium attendees can learn something<br />
where others spend their holidays.<br />
Lisbon yesterday and today<br />
Lisbon, the Portuguese capital, has 560,000 inhabitants<br />
within its administrative limits (more than<br />
2 million overall) and is known as the City of the<br />
Seven Hills. From its commercial port in the bay of<br />
the Tejo River, Portuguese sailors set out to conquer<br />
the world starting in the late 15th century. The<br />
city has many sights worth seeing – starting with<br />
the Belem Tower, the widely visible defence tower<br />
dating back to the 16th century and certainly not<br />
ending with the fascinating “azulejos”, the blue tiles<br />
that still adorn many houses in the old city, originally<br />
introduced to Portugal by the Moors.<br />
Portugal’s Estoril coast<br />
An exclusive resort long frequented by the local<br />
nobility, Estoril is less than 30 km away from Lisbon –<br />
about half an hour by car. Given this short distance, it<br />
is probably a good idea to spend the entire time at<br />
the Palácio Estoril Golf & Spa, riding in to Lisbon only<br />
for the symposium itself. The Palácio is probably the<br />
most well-known and certainly the most beautiful<br />
hotel in town and has a five-star rating. The rooms<br />
with their marble bathrooms are lavishly and comfortably<br />
furnished. The hotel itself was built in 1930 and
Elegance and<br />
refinement<br />
in the most<br />
beautiful hotel<br />
in Estoril: The<br />
Palácio Estoril.<br />
radiates elegance and refinement. It was the refuge<br />
for many eminent personalities during World War II.<br />
Famous films were shot here as well, including a James<br />
Bond film, “On Her Majesty’s Secret Service”. The beach<br />
is only 200 m away from the hotel with its 161 rooms<br />
and its renowned restaurants featuring local specialties<br />
and international cuisine, a gourmet grill, piano<br />
bar, ocean water swimming pool and sun terrace. During<br />
World War II, Bar Estoril was a popular meeting<br />
point for spies. Today the place offers an atmosphere<br />
of classical charm and a view of the poolside gardens.<br />
The hotel’s own golf course, the Parcour Golf do<br />
Estoril, is located only 5 km northeast of the hotel.<br />
One of Europe’s largest casinos is within walking distance,<br />
with one of the most beautiful beaches, Praia<br />
do Tamariz, directly adjacent.<br />
Schedule of events<br />
<strong>EDI</strong> 21<br />
Participants and their families can enjoy a one-week<br />
stay in rooms with a highly exclusive ambience<br />
(breakfast included) at the Palácio Estoril Golf & Spa<br />
in Estoril or, alternatively, two nights (10 – 12 June) at<br />
the Real Palácio in Lisbon and then move to the<br />
Palácio Estoril. The arrival date is 10 June. The international<br />
symposium and dental exhibition will take<br />
place at the Pestana in Lisbon on Saturday, 11 June.<br />
An interactive week of continuing education begins<br />
at the Palácio Estoril Golf & Spa on Monday, 13 June,<br />
and continues through Thursday, 16 June. Friday,<br />
17 June, will be the day of departure.<br />
AWU
22<br />
<strong>EDI</strong><br />
<strong>EDI</strong> News<br />
Symposium Saturday, 11 June 2011; Pestana Hotel, Lisbon<br />
08:45 – 09:00 am Professor Antonio Felino, Porto<br />
Christian Berger, Kempten<br />
✁<br />
Fax your registration to:<br />
+49 89 72069023<br />
or send an e-mail to:<br />
office-munich@bdizedi.org<br />
or send to<br />
BDIZ <strong>EDI</strong><br />
Lipowskystr. 12<br />
81373 Munich<br />
Germany<br />
Welcoming address<br />
09:00 – 09:25 am Professor João Carames, Lisbon Rehabilitation of the atrophic maxilla: New strategies<br />
09:30 – 09:55 am Dr Nuno Braz de Oliveira, Sesimbra Management of complex cases<br />
10:00 – 10:25 am Dr Roque Braz de Oliveira, Sesimbra Multi-detail management of the anterior maxillary segment – clinical cases and<br />
science<br />
10:30 – 11:00 am Coffee break · Exhibition visit<br />
11:00 – 11:25 am Dr Ricardo Faria Almeida, Porto Immediate implants and immediate function in the aesthetic zone – new challenges<br />
11:30 – 11:55 am Dr Francisco Salvado, Lisbon Bone regeneration: Bone, biomaterials and tissue engineering<br />
12:00noon–12:25 pm Professor Hakan Özyuvaci, Istanbul Reflections on 3D imaging in oral surgery and implantology<br />
12:30 – 12:55 pm Professor Joachim E. Zöller, Cologne Augmentation: Different techniques and different materials – high risk, low risk<br />
1:00 – 2:30 pm Lunch · Exhibition visit<br />
2:30 – 2:55 pm Dr Detlef Hildebrand, Berlin The TEAM-BERLIN-CONCEPT: Planning and implementation of implants<br />
in complex cases – a team approach<br />
3:00 – 3:25 pm Professor Antonio Felino, Porto The importance of bone-preserving surgical techniques for subsequent<br />
implantation procedures – clinical practice<br />
3:30 – 3:55 pm Coffee break · Exhibition visit<br />
3:55 – 4:20 pm Dr Dirk U. Duddeck, Cologne Comparative investigation of various implant surfaces by SEM analysis –<br />
a surprise in the land of microns<br />
4:25 – 4:55 pm Professor João Carlos Ramos, Coimbra Immediate implants: From investigation to clinical practice<br />
5:00 – 5:30 pm Professor Antonio Felino<br />
Professor Joachim E. Zöller<br />
Final Discussion<br />
Interactive education at the Palácio Estoril in Estoril: Monday, 13 June to Thursday, 16 June.<br />
Registration<br />
5th BDIZ <strong>EDI</strong> Mediterranean Symposium (11 June 2011), Pestana Hotel, Lisbon<br />
Continuing-education week, Palácio Estoril Golf & Spa Hotel, Estoril, 13 to 16 June<br />
Travel and accommodation:<br />
10–12 June Lisbon: Hotel Real Palácio ***** / with breakfast<br />
Doubles: € 78 per person per night, Singles: € 139 per person per night<br />
12–17 June Estoril: Hotel Palácio Estoril ***** / with breakfast<br />
Doubles: € 130 per person per night, Singles: €230 per person per night<br />
For reduced rates for children and prices for extension days, please inquire.<br />
Event charge<br />
Until 15 April € 480, after 16 April € 680<br />
(including the symposium and one week of interactive continuing education)<br />
Booking through a different travel agency or registering for the continuing-education course only<br />
is subject to a € 500 surcharge.<br />
For travel and course details please visit www.bdizedi.org (Events).<br />
I want to attend the 5th BDIZ <strong>EDI</strong> Mediterranean Symposium (10–17 June 2011). Please contact me.<br />
Surname, Firstname<br />
Street, Number<br />
Postal Code, City<br />
Contact, Phone, E-Mail<br />
Signature<br />
Each participant will be awarded 8 CE points per day.
Planmeca sets new standards with<br />
world’s fi rst dental unit integrated intraoral scanner<br />
for open connectivity to various CAD/CAM systems.<br />
We would like to invite you to explore the dentistry in new dimensions – see the perfect combination of digital<br />
intraoral scan, CBVT and 3D facial photo datasets in one 3D image. This digital perfection enables you to study<br />
patient’s complete anatomy in detail, plan and utilise open interface with modern CAD/CAM systems according<br />
to your needs. Now you can be one of the pioneering specialists, whether you are an implantologist, endodontist,<br />
periodontist, orthodontist or maxillofacial surgeon. The new era of dentistry is reality. It’s your decision.<br />
Planmeca Oy, Asentajankatu 6, 00880 Helsinki, Finland<br />
tel. +358 20 7795 500, fax +358 20 7795 555, sales@planmeca.com, www.planmeca.com<br />
1971 - 2011
24<br />
<strong>EDI</strong><br />
<strong>EDI</strong> News<br />
Teamwork and collaboration will be the central issue<br />
at this congress – collaboration between the two<br />
organizing associations, of course, but mainly collaboration<br />
between the patient, dental nurses and assistants,<br />
dental technicians and dentists. Professor<br />
Joachim E. Zöller, Professor Georg-H. Nentwig, Dr Georg<br />
Bayer and Christian Berger have also collaborated – on<br />
creating the schedule of events and on selecting the<br />
speakers. The result is a top-notch program with international<br />
contributions that neither dentists nor their<br />
teams nor dental technicians should miss.<br />
The presentations during the two congress days<br />
include the entire range of what is important for oral<br />
implantologists and their teams. The congress will<br />
be kicked off on Friday by the Young Implantologists<br />
Forum and the first industry workshop session. The<br />
main podium for all attendees will feature Professor<br />
Wilfried Wagner discussing serious risks that can be<br />
present in the oral cavity, e.g. in osteoporosis patients,<br />
and the treatment providers must beware of. Dr Jörg<br />
Neugebauer will present information about the<br />
application, cost and benefits of CBCT in the dental<br />
practice. Marketing professional Professor Gerhard F.<br />
Riegl will speak about the factors that make a dental<br />
office or clinic a success. Dr Gerd Körner and Dr Josef<br />
Diemer will report on periodontal treatment prior to<br />
implant placement and on tooth preservation from<br />
the endodontist’s point of view. BDIZ <strong>EDI</strong> legal advisor<br />
Dr Thomas Ratajczak will inform the audience on<br />
issues to keep in mind when informing patients of<br />
available treatment alternatives. After a final discussion<br />
on this set of topics, a second industry workshop<br />
session will conclude Friday’s business.<br />
15th BDIZ <strong>EDI</strong> Symposium · 8th International<br />
Annual Congress of the DGOI<br />
Implantology and<br />
the dental team<br />
Two organizers, one event: All systems are go for the joint congress of BDIZ <strong>EDI</strong> and DGOI in Munich on 16/17 September 2011.<br />
All spotlights are highlighting “Implantology and the dental team”. The five-star Hotel Sofitel Munich Bayerpost – next to the<br />
central railway station – has once again been selected as the pivotal point for dentists, dental nurses and assistants, dental<br />
technicians, the dental industry and of course the speakers at this two-day continuing-education event. In anticipation of<br />
Oktoberfest, scheduled to start the same weekend, BDIZ <strong>EDI</strong> and DGOI would love to see all attendees visit the special<br />
pre-Oktoberfest held in the Löwenbräukeller’s Oktoberfest “marquee”.<br />
Oktoberfest marquee<br />
The evening will be rustic: In anticipation of Oktoberfest,<br />
scheduled to start the same weekend, BDIZ <strong>EDI</strong><br />
and DGOI would love to see all attendees visit the<br />
Oktoberfest “marquee“ at Löwenbräukeller on centrally<br />
located Stigelmaierplatz. Pre-Oktoberfest means<br />
that we will be getting a head start by one day –<br />
the day before the traditional tapping of the barrel<br />
by Munich’s mayor. Many Oktoberfest aficionados can<br />
hardly wait to celebrate in the traditional manner<br />
and to savour the classic Oktoberfest atmosphere –<br />
enjoying Bavarian music and cabaret artists, and not<br />
least hearty food and drink.<br />
The implantological world<br />
assembles in Munich<br />
The entire world – it is said – will assemble in Munich<br />
at Oktoberfest. The implantological world, too, will<br />
assemble in Munich that weekend – with global<br />
speakers adding international flair: Speakers Dr Carl<br />
Misch (USA), Dr Eduardo Anitua (Spain) and Dr Maurice<br />
Salama (USA) will kick off Saturday’s program by<br />
discussing immediate vs. delayed implant placement,<br />
results achieved with short implants and orthodontic<br />
treatment before and after implant placement.<br />
Hard-tissue and soft-tissue aspects will be highlighted<br />
by Professor Joachim E. Zöller and Dr Stefan<br />
Reinhardt in their presentations on differential indications<br />
of augmentation techniques and materials and<br />
on new aspects in soft-tissue management, respectively.<br />
The presentations after lunch will once again
Looking For<br />
Efficiency?<br />
Encode is a registered trademark and Providing Solutions - One Patient At A Time and design<br />
are trademarks of BIOMET 3i LLC. BIOMET 3i and design are trademarks of BIOMET, Inc.<br />
©2011 BIOMET 3i LLC. All rights reserved.<br />
Enhanced<br />
Knowledge<br />
Comprehensive<br />
Treatment<br />
Business<br />
Excellence<br />
Introducing ’s Encode ® Impression on System For Improved Efficiency<br />
The Patient Friendly Solution:<br />
�� �����������������������������������������������<br />
For Implant Level Impressions<br />
�� ������������������<br />
�� �����������������������������������������<br />
Preserve The Soft Tissue<br />
Providing Clinicians��������������������������������������� ® Impression System<br />
�� �����������������������������������<br />
����������3i�����������������<br />
�����������������������������������������������<br />
�����������������������������������������<br />
��������������������������������������<br />
In Titanium or Zirconia<br />
Follow us on:<br />
�������������<br />
The Encode ®<br />
Impression<br />
System!<br />
Come and visit us at<br />
the 2011 IDS<br />
March 22-26, 2011<br />
Koelnmesse<br />
Hall 4.2<br />
Aisle J Nº039 - Aisle G Nº030<br />
To Improve Your Efficiency With The Encode® Impression System,<br />
Contact Your Local Sales Representative Today.<br />
Europe, Middle East, Africa Headquarters: +34-93-470-55-00<br />
Or visit us online at www.biomet3i.com<br />
���������������<br />
Smartphone!<br />
���������������������������<br />
������������������������<br />
���������������<br />
reader installed.<br />
ENCODE_ADV_B3I_EMEA(A4).indd 1 03/02/11 16:50
26<br />
<strong>EDI</strong><br />
<strong>EDI</strong> News<br />
Pre-Oktoberfest<br />
A contiguous section of seats has been reserved<br />
for BDIZ <strong>EDI</strong> and DGOI in the “Wiesn Marquee” at<br />
Löwenbräukeller on 16 September 2011. The ticket<br />
price of €38 p.p. includes a voucher for food and<br />
drink worth €16. While you may expect to see<br />
some guests in traditional Bavarian attire, there is<br />
no specific dress code. Sturdy clothing is recommended.<br />
address the entire team. Dr Fred Bergmann will shed<br />
some light on the interaction between surgical and<br />
prosthetic issues and discuss patient management.<br />
Professor Daniel Edelhoff will discuss whether CAD/<br />
CAM is ready to replace conventional impressions.<br />
Implantologists and dental technicians alike are also<br />
the presumptive audience of the presentation by<br />
Peter Finke on potential laboratory and prosthodontic<br />
complications.<br />
Late Saturday afternoon also has something in<br />
store for everyone: managing complications in<br />
referred patients (Dr Michael Stiller), “correct” billing<br />
for implantological treatments (Dr Hans-Joachim<br />
Friday, 16 September 2011<br />
Scientific program<br />
Implantology and the dental team<br />
08:00 – 10:00 am Young Implantologists Podium<br />
10:00 – 10:15 am Discussion<br />
10:15 – 10:30 am Coffee break · Exhibition visit<br />
10:30 am – 12:00 noon Workshop Session I<br />
12:00 noon – 1:00 pm Buffet lunch · Exhibition visit<br />
1:00 – 1:15 pm Welcome and introduction<br />
Dr Georg Bayer, President of DGOI, and<br />
Christian Berger, President of BDIZ <strong>EDI</strong><br />
1:15 – 2:00 pm Risk factors in the oral cavity<br />
(HIV, bisphosphonates)<br />
Professor Wilfried Wagner, Mainz<br />
2:00 – 2:30 pm CBCT – when, which, why, how much?<br />
Dr Jörg Neugebauer, Landsberg am Lech<br />
2:30 – 3:00 pm Teamwork at the successful<br />
dental office<br />
Professor Gerhard F. Riegl, Augsburg<br />
3:00 – 3:15 pm Discussion<br />
Nickenig) and aesthetic issues to be addressed by<br />
dentists in the laboratory (Dr Paul Weigl). The final<br />
discussion, too, is something to look forward to,<br />
because it will almost certainly address some questions<br />
related to the German fee schedule, GOZ.<br />
With this joint congress, BDIZ <strong>EDI</strong> and DGOI wish<br />
to send a signal in favour of deceleration, given the<br />
madly spinning carousel of implantological congresses<br />
and events in Germany. Dentists have long<br />
since lost track of the multitude of dates and offerings;<br />
the industry, too, has been complaining about<br />
the excessive number of events.<br />
AWU<br />
3:15 – 3:45 pm Coffee break · Exhibition visit<br />
BDIZ <strong>EDI</strong> and<br />
DGOI would<br />
love to see all<br />
attendees visit<br />
the special<br />
pre-Oktoberfest.<br />
3:45 – 4:15 pm Pre-implantological periodontics<br />
Dr Gerd Körner, Bielefeld<br />
4:15 – 4:45 pm Informing patients about treatment<br />
alternatives<br />
Dr Thomas Ratajczak, Sindelfingen<br />
4:45 – 5:15 pm Tooth preservation from an<br />
endodontist’s point of view<br />
Dr Josef Diemer, Meckenbeuren<br />
5:15 – 5:30 pm Final discussion<br />
Professor Georg-Hubertus Nentwig,<br />
Frankfurt, Professor Joachim E. Zöller,<br />
Cologne<br />
5:30 – 7:00 pm Workshop Session II<br />
Evening<br />
8:00 pm Kicking off Oktoberfest:<br />
Bavarian night at Löwenbräukeller<br />
Bavarian food and drink, music<br />
and comedy at the 13th “Wiesnzelt”<br />
marquee
Let’s get to work.<br />
True working results with the true all-in-one<br />
Orthopantomograph ®<br />
Orthopantomograph ®<br />
OC200 D VT<br />
The only original Orthopantomograph ®<br />
with new Core Lateral Ceph imaging<br />
program. Same proven superb image quality as before with up to 68% less radiation.<br />
All the important imaging tools you need in one unit – including Volumetric Tomography.<br />
Learn more at www.instrumentariumdental.com<br />
IDS Cologne 2011, Our stand in Hall 11.2, Aisle P, 050<br />
www.instrumentariumdental.com<br />
ID_OC200_D_add_<strong>EDI</strong>_2011.indd 1 24.2.2011 16:51:53
28<br />
✁<br />
<strong>EDI</strong><br />
<strong>EDI</strong> News<br />
Saturday, 17 September 2011<br />
Scientific program<br />
Implantology and the dental team<br />
08:30 – 08:45 am Welcome and introduction<br />
Professor Georg-Hubertus Nentwig,<br />
Professor Joachim E. Zöller<br />
08:45 – 09:30 am Immediate vs. delayed implant<br />
placement<br />
Dr Carl Misch, Beverly Hills/USA<br />
09:30 – 10:15 am Performance of short implants –<br />
evidence-based?<br />
Dr Eduardo Anitua, Vitoria/Spain<br />
10:15 – 11:00 am Orthodontic treatment before and<br />
after implant placement<br />
Dr Maurice Salama, Atlanta/USA<br />
11:00 – 11:15 am Discussion<br />
11:15 – 11:45 am Coffee break · Exhibition visit<br />
11:45 am – 12:30 pm Differential indications of augmentation<br />
techniques and materials<br />
Professor Joachim E. Zöller, Cologne<br />
12:30 – 12:45 pm New aspects of soft-tissue<br />
management<br />
Dr Stefan Reinhardt, Münster<br />
12:45 – 1:00 pm Discussion<br />
Please register via fax:<br />
+49 8243 9692-55<br />
or online: www.bdiz.dgoi.teamwork-media.de<br />
or by mail<br />
teamwork media GmbH<br />
Hauptstraße 1<br />
86925 Fuchstal<br />
Germany<br />
Registration<br />
1:00 – 2:00 pm Lunch break · Exhibition visit<br />
2:00 – 2:30 pm Surgery and prosthetics as team<br />
players: Patient management<br />
Dr Fred Bergmann, Viernheim<br />
2:30 – 3:00 pm Will CAD/CAM replace conventional<br />
impressions?<br />
Professor Daniel Edelhoff, Munich<br />
3:00 – 3:30 pm Laboratory and prosthodontic<br />
complications and liability issues<br />
Peter Finke, Erlangen<br />
3:30 – 3:45 pm Discussion<br />
3:45 – 4:15 pm Coffee break · Exhibition visit<br />
4:15 – 4:45 pm Managing complications in referred<br />
patients<br />
Dr Michael Stiller, Berlin<br />
4:45 – 5:15 pm “Correct” billing for implantological<br />
treatments<br />
Dr Hans-Joachim Nickenig, Cologne<br />
5:15 – 5:45 pm Laboratory-office teamwork:<br />
Aesthetics<br />
Dr Paul Weigl, Frankfurt<br />
5:45 – 6:00 pm Final discussion<br />
Dr Georg Bayer, Christian Berger<br />
Symposium<br />
Members of BDIZ <strong>EDI</strong>: € 350 (after 1 July: € 420)<br />
Non-members: € 450 (after 1 July: € 520)<br />
Assistant dentists: € 280 (after 1 July: € 350)<br />
Dental students: € 200 (after 1 July: € 270)<br />
Program for dental assistants<br />
Nurses, assistants: € 150 (after 1 July: € 180)<br />
Pre-Oktoberfest night: ______ tickets @ € 38 p.p. = € __________<br />
(subject to availability)<br />
Family name and given name<br />
Street address<br />
Postal code and city<br />
Contact/Phone/E-Mail<br />
Date and signature
Diagnostic<br />
performance!<br />
Versatile. Easy. Eff ective.<br />
Come to visit us at IDS Cologne<br />
Hall 11.2. Aisle N Booth 050<br />
Digital imaging made easy<br />
www.soredex.com<br />
SCANORA® 3D low dose digital cone<br />
beam unit featuring real panoramic<br />
imaging.<br />
The SCANORA® 3D contains the newest<br />
results of our continuous development<br />
work.<br />
With its various 3D image volumes<br />
and dedicated panoramic imaging the<br />
SCANORA® 3D system off ers a perfect<br />
solution for your daily imaging tasks.<br />
The fully motorized seat, easy ClearTouch<br />
control panel and ergonomic patient<br />
stabilization ensure fast workfl ow and<br />
superb results.<br />
Indispensable in implant dentistry and<br />
dento-maxillo-facial diagnostics.<br />
Manufacturer: SOREDEX<br />
P.O.Box 148, 04301 TUUSULA, Finland<br />
������������������������������������������<br />
Advert_template_2011_2_A4.indd 1 24.2.2011 16:36:19
30<br />
<strong>EDI</strong><br />
<strong>EDI</strong> News<br />
“Academy Award” of the German printing world<br />
PrintStar 2010 nomination<br />
for BDIZ <strong>EDI</strong> konkret<br />
Every year, the German printing industry offers its PrintStar industry awards to the top printed products and printing technologies<br />
in Germany. Last year, the BDIZ <strong>EDI</strong> member publication, BDIZ <strong>EDI</strong> konkret, was among the nominees for the Newspapers and<br />
Magazines category, placing it among Germany’s selected Top Ten. BDIZ <strong>EDI</strong> konkret is the German-language equivalent of the<br />
English-language <strong>EDI</strong> Journal. Both implantological journals are published by BDIZ <strong>EDI</strong> through teamwork media four times a year.<br />
The patron of this innovation award is Rainer Brüderle,<br />
German Minister for Economics and Technology. This<br />
is the first time that an implantological publication<br />
is included among the nominees. For example, the<br />
PrintStar 2010 competitors of BDIZ <strong>EDI</strong> konkret<br />
included such well-known German general-interest<br />
publications as Elle and Freundin by Burda and Cicero<br />
by Ringier. Ultimately, Elle prevailed for first prize.<br />
Joint runners-up were Cicero and novum – World of<br />
Graphic Design by the publisher of Augsburger All -<br />
gemeine. The third prize was awarded to Q-Querdenker-Magazin<br />
printed by Gotteswinter in Munich,<br />
which also prints BDIZ <strong>EDI</strong> konkret.<br />
“We are proud that BDIZ <strong>EDI</strong> konkret as a professional<br />
dental journal has achieved this level of visibility<br />
amid the enormous number of glossy magazines<br />
published in Germany”, said BDIZ <strong>EDI</strong> President Christian<br />
Berger as he reflected on the PrintStar 2010 nomination.<br />
He thanked the publishers, teamwork media,<br />
the printers, Gotteswinter, and the Assistant Editorin-Chief,<br />
Anita Wuttke, as representative of the entire<br />
editorial team. According to Berger, this shows the<br />
high expectations BDIZ <strong>EDI</strong> has of its member publication<br />
in terms of editorial content, graphic design<br />
and printing technology. “For BDIZ <strong>EDI</strong> konkret, this<br />
means a terrific boost”, agreed Ralf Suckert, the publisher<br />
and managing director of the teamwork media<br />
publishing group, Fuchstal. This nomination, he said,<br />
bears witness to the high level of competence in prepress<br />
(teamwork media) and printing (Gotteswinter).<br />
PrintStar, the innovation award of the German printing<br />
industry, is offered each year in the categories of<br />
printed products, marketing and technology, evaluating<br />
innovative implementation and overall composition.<br />
Considered the most highly renowned printing<br />
award in Germany, it is awarded by a professional<br />
jury that depending on the prize category in question,<br />
welcomes entries from print offices, agencies,<br />
photographic studios, publishers, prepress houses<br />
and posttest companies as well as print buyers with<br />
their corporate headquarters or branch offices in Germany,<br />
as well as German vocational or technical colleges<br />
and the international supply industry.<br />
AWU
<strong>EDI</strong><br />
<strong>EDI</strong> News<br />
Career notes: Dr Dirk U. Duddeck<br />
BDIZ <strong>EDI</strong><br />
Administrator,<br />
Bonn office<br />
Dr Dirk U. Duddeck, dentist from Cologne, has been<br />
supporting BDIZ <strong>EDI</strong> in his new capacity as adminis-<br />
trator of the association’s Bonn office since mid-2010.<br />
Dr Dirk U. Duddeck<br />
He coordinates and optimizes administrative procedures<br />
in terms of quality management, serving as<br />
liaison between association members, the BDIZ <strong>EDI</strong><br />
office and the Board of Directors. In addition, he has<br />
been an active member of the BDIZ <strong>EDI</strong> Qualification<br />
and Registration Committee. He conducted and<br />
supervised the joint study by BDIZ <strong>EDI</strong> and the University<br />
of Cologne on contamination and residue<br />
on sterile-wrapped implants and is currently<br />
engaged in conducting the follow-up study. Since<br />
2007, Dr Duddeck has been a research assistant at<br />
the Interdisciplinary Policlinic for Oral Surgery and<br />
Implantology and the Department of Oral and Maxillofacial<br />
Plastic Surgery at the University of Cologne.<br />
In 2009, he took on the additional responsibility as<br />
department head and coordinator of dental studies<br />
in the Dean of Studies office at the University of<br />
Cologne and project manager of a longitudinal curriculum<br />
on social and communicative competence<br />
for dental students (Innovationen in Lehre und Studium,<br />
LSK-DENT). Previously, Dr Duddeck had been program<br />
director and department head for a medical<br />
publisher; from 1999 to 2006 he had been one of the<br />
founding partners of a leading continuing-education<br />
firm and consultancy.<br />
AWU<br />
31<br />
Implant-retained<br />
Dentures<br />
Suprastructure<br />
porcelain veneered<br />
Suprastructure latched<br />
to mesostructures<br />
Non precious metal TITANIUM<br />
Passive fi t by using SAE spark erosion<br />
Long-term osseointegration<br />
20 years of experience!<br />
Your competent partner<br />
for implant-retained dentures<br />
Clinical photographs:<br />
Dr. E. Eisenmann,<br />
Free University Berlin,<br />
Benjamin Franklin Medical Center<br />
Catalogue<br />
Implant-retained Dentures<br />
Dentist and Patient<br />
information<br />
including denturescost-estimates<br />
Please order for free!<br />
Rübeling Dental-Labor<br />
Langener Landstr. 173 · 27580 Bremerhaven · Germany<br />
Phone: +49 471 / 984 87-0 · Fax: +49 471 / 984 87-44<br />
E-Mail: info@ruebeling.de · www.ruebeling.de
32<br />
<strong>EDI</strong><br />
<strong>EDI</strong> News<br />
20th International Expert Symposium for Regenerative Methods<br />
in Medicine and Dentistry<br />
Biomaterials under scrutiny<br />
The Expert Symposium for Regenerative Methods in Medicine and Dentistry was held in 2010 – the 20th of<br />
these symposiums. More than 150 dentists attended the event on Fuerteventura. This time the symposium<br />
was held under the motto of “Implantology and biomaterials: Innovation in and beyond surgery”.<br />
Professor Joachim E. Zöller had once again succeeded in attracting international speakers to the Robinson<br />
Club Esquinzo Playa on Fuerteventura to stimulate a lively exchange of views with and among attendees.<br />
The – interesting yet controversial – topic selected for the anniversary symposium was that of biomaterials.<br />
Biomaterials have been the subject of much controversy<br />
among experts – and this became evident on<br />
Fuerteventura as well. On the one hand, faculty members<br />
of the University of Cologne and Dresden presented<br />
a number of scientific studies offering supportive<br />
evidence for various materials. On the other hand,<br />
there were vocal groups whose members critically<br />
remarked that not all biomaterials pushed into the<br />
market with the help of enormous marketing budgets<br />
actually meet every day clinical requirements.<br />
Different requirements<br />
The symposium also showed that the category of<br />
biomaterials not only includes the typical bone<br />
replacement materials but must also be related to<br />
the properties of the implants themselves, prosthetic<br />
components and products that safeguard therapeutic<br />
success in the event of inflammatory changes<br />
in the peri-implant tissues.<br />
A special effort was made at the symposium to<br />
present not only the various materials and products<br />
shown by the exhibitors on-site. Rather, speakers<br />
explained clinical treatment procedures related to<br />
the biomaterials, providing highly relevant information<br />
for clinical practice – an approach that was clearly<br />
appreciated by the participants.<br />
As in previous years, current developments in dentistry,<br />
and especially the use of digital imaging procedures,<br />
was given ample room on the agenda. In<br />
particular the possibilities afforded by digital impressions<br />
and the use of the relevant techniques in planning<br />
implant treatment and restorative procedures<br />
were impressively highlighted by several speakers.<br />
Even though the number of attendees in 2010 had<br />
again been higher than the year before, the cher-<br />
ished informal nature of the event had remained<br />
intact. An intensive exchange of ideas above and<br />
beyond the scientific program has been a focal goal<br />
during the past 20 years, and this approach was<br />
further supported by the hotel’s facilities. Attendees<br />
took part in the physical activities that were<br />
an important aspect of the social part of the symposium,<br />
true to the idea of “a healthy mind in a healthy<br />
body”.<br />
The dental industry, too, had been very interested<br />
in being present on Fuerteventura in order to intensify<br />
its contacts with the participants. However, this<br />
obvious interest was in no way detrimental to the<br />
event, which – being spread out over a full week –<br />
was certainly not dominated by consultations in a<br />
hectic atmosphere where one of the sides was dead<br />
set to “persuade” the other. Rather, there was a serious<br />
exchange of ideas in a relaxed atmosphere.<br />
The 21st Expert Symposium will be focusing on<br />
basic procedures and surgical techniques. It will be<br />
held from 27 October to 3 November 2011.<br />
NEU
Want More?<br />
Broaden Your<br />
Options<br />
Introducing<br />
*Cobalt Chrome available in Europe only.<br />
NEW<br />
The Solution Designed To Provide Clinicians With<br />
More Options:<br />
�� Copings And Frameworks Now Available Through<br />
Dental Laboratories<br />
�������������������������������������������������������������<br />
Tooth Restorations<br />
3i is a registered trademark and Providing Solutions - One Patient At A Time and design are<br />
trademarks of BIOMET 3i LLC. BIOMET 3i and design are trademarks of BIOMET, Inc. Renishaw<br />
is a registered trademark and apply innovation and incise are trademarks of Renishaw plc.<br />
©2011 BIOMET 3i LLC. All rights reserved.<br />
Enhanced<br />
Knowledge<br />
Providing Clinicians One Solution At A Time With<br />
�������������������������������� /<br />
�� Copings And Frameworks Available In Zirconia And Cobalt Chrome*<br />
������������������������������������������������������������<br />
Comprehensive<br />
Treatment<br />
Business Business<br />
Excellence<br />
Excellence<br />
To Broaden Your Options With , , Contact Your<br />
Local Sales Representative Today!<br />
Europe, Middle East, Africa Headquarters: +34-93-470-55-00<br />
Or visit us online at www.biomet3i.com<br />
3I INCISE CLINICIANS_ADV_B3I_<strong>EDI</strong>(A4).indd 1 03/02/11 16:51
34<br />
<strong>EDI</strong><br />
<strong>EDI</strong> News<br />
Third anniversary of the von Willebrand syndrome (vWS) network<br />
Haemophilia’s little sister<br />
von Willebrand syndrome (vWS) or von Willebrand disease (vWD) is a mainly hereditary coagulation abnormality which,<br />
although relatively frequent, is not widely known and difficult to recognize. Not infrequently it is not diagnosed until a patient<br />
experiences acute bleeding, e.g. during dental therapy or after childbirth. The disease arises from a qualitative or quantitative<br />
deficiency of von Willebrand factor (vWF), the largest protein in the human body. In 2008, Network vWS was founded by<br />
various German associations and interest groups, including the German Haemophilia Society (Deutsche Hämophiliegesellschaft,<br />
DHG), the Haemophilia Special Interest Groups (Interessengemeinschaft Hämophiler, IGH), the Professional Association of<br />
Gynaecologists (Berufsverband der Frauenärzte, BVF) and BDIZ <strong>EDI</strong>. The network presented a synopsis of its first three years<br />
of activity at a press conference in Hamburg.<br />
“Haemophilia is well known, even though it is rare.<br />
von Willebrand syndrome is generally unknown<br />
despite the fact that it affects approximately<br />
800,000 men and women in Germany.” This astonishing<br />
fact was pointed out by Professor Reinhard<br />
Schneppenheim, Medical Director of the Department<br />
of Paediatric Haematology and Oncology at the<br />
Eppendorf University Hospital in Hamburg. Classic<br />
haemophilia – a coagulation factor VIII or IX deficiency,<br />
haemophilia A and B, respectively – affects<br />
only 6,000 men in Germany.<br />
Difficult to diagnose<br />
vWS is mostly characterized by less prominent, diffuse<br />
symptoms often not associated with each other<br />
by patients or even by many specialist physicians, as<br />
Professor Schneppenheim reported: “In children, vWS<br />
manifests itself by a pronounced tendency toward<br />
haematomas and nosebleeds. Type 3, the most severe<br />
form of the disease, may also include bleeding in the<br />
joints (haemarthroses) and muscles, more rarely in<br />
the cerebral area, as in the case of haemophilia. All<br />
incarnations of vWS are characterized by a tendency<br />
toward mucosal bleeding, e.g. following a tonsillectomy<br />
or polypectomy. Protracted bleeding suggesting<br />
vWS may also be seen during exfoliation.”<br />
Dr Klaus König (Steinbach), gynaecologist in private<br />
practice representing the Professional Association<br />
of Gynaecologists, reported that extended or<br />
very strong menstrual bleeding in women may be<br />
an indication of the syndrome. It is frequently not<br />
diagnosed since the affected women compare<br />
themselves with their mothers, sisters or aunts, who<br />
are often themselves affected by this syndrome and<br />
exhibit similar symptoms: “Following childbirth,<br />
these women often experience very strong bleeding,<br />
which is extremely difficult to stop.”<br />
Haematomas, nosebleeds, strong and extended<br />
menstrual bleeding, post-bleeding following dental<br />
treatment or surgery – these are the symptoms that<br />
Dr Günter Auerswald (Bremen) from the special outpatient<br />
Department for Thrombosis and Haemostatic<br />
Disorders at Bremen-Mitte hospital associates with<br />
vWS. All three speakers, Schneppenheim, König and<br />
Auerswald, admitted that the syndrome was difficult<br />
to diagnose. This diagnosis involves a three-level series<br />
of laboratory examination. There are several clinical<br />
centres in Germany specializing in this diagnosis.<br />
Treatment options<br />
Professor Schneppenheim described the treatment<br />
options as follows: If von Willebrand factor – required<br />
for stabilizing and transporting clotting factor VIII,<br />
collagen binding (in the case of injured blood vessels)<br />
The von Willebrand<br />
disease<br />
arises from a<br />
qualitative or<br />
quantitative<br />
deficiency of<br />
von Willebrand<br />
factor. The diagnosis<br />
involves a<br />
three-level series<br />
of laboratory<br />
examination.
and platelet binding – is missing, it could be re -<br />
placed by concentrates. Releasing the factor from<br />
depots within the body by administering desmopressin<br />
(DDAVP) is another treatment option that is<br />
primarily indicated for the milder type 1 of the disease,<br />
but also for some patients with type 2. (Editor’s<br />
note: DDAVP is a hormone that improves haemostasis<br />
by increasing plasma coagulation factor VIII and<br />
vWF levels.) In all cases, these patients should be<br />
trialled on DDAVP for effectiveness of this therapy.<br />
“These patients should never undergo outpatient<br />
surgery, but should be treated surgically only at centres<br />
with demonstrable experience in treating vWS”,<br />
said Schneppenheim.<br />
Free network use<br />
The main information platform of Network vWS, initiated<br />
by CSL Behring, is its website, with marketing<br />
assistance provided by Medical Consulting Group<br />
(MCG), Düsseldorf. MCG presented the results of<br />
the work of the past three years to representatives<br />
of all network associations and working groups: The<br />
media for the general public had achieved a print run<br />
<strong>EDI</strong><br />
<strong>EDI</strong> News<br />
of 63 million, while the media for a professional audience<br />
had achieved a print run of 2.5 million. Cinema<br />
advertisement, print advertisement, the web platform,<br />
press conferences, continuing-education seminars<br />
for physicians, self-test for potential patients,<br />
an atlas to support patient-physician consultations –<br />
all these have contributed toward informing the<br />
public of the syndrome and sensitizing the medical<br />
community.<br />
In a brainstorming session, the attendees –<br />
including BDIZ <strong>EDI</strong> President Christian Berger – discussed<br />
potential directions for the future activities<br />
of the network. They listened to suggestions for<br />
patient information via in-office CCTV as well as<br />
to demands for interactive anamnestic forms to be<br />
downloaded from the web. Other topics were apprehensions<br />
of over-diagnosis by medical labs and the<br />
right amount of sensitivity in preparing information<br />
for the public. Christian Berger pointed out that<br />
dentists, who meet potential vWS patients in their<br />
offices and at schools at a very early stage, might<br />
play an important part in early recognition of this<br />
disease.<br />
AWU<br />
Optimal Bone Regeneration and<br />
Implant Osseointegration<br />
– with Tigran TM PTG (Porous Titanium Granules)<br />
TTIGRAN<br />
IGRAN<br />
TTECHNOLOGIES<br />
ECHNO<br />
LOGIES<br />
AB<br />
MMEDEON<br />
EDEON<br />
SCIENCE SCIEN<br />
CE<br />
PARK, PARK,<br />
205 12<br />
MALMÖ, MALMÖ,<br />
SWEDEN SWEDE<br />
N<br />
WWWW.TIGRAN.SE WW<br />
TIGR<br />
AN<br />
SE<br />
+46 + 46<br />
40 4 0 693<br />
92<br />
70<br />
.<br />
.<br />
Predictable P redict<br />
ctable<br />
rresults<br />
esults<br />
DDoes<br />
oes<br />
nnot<br />
ot<br />
rresorb<br />
eso<br />
r rb<br />
aand<br />
nd<br />
ttherefore<br />
herefore<br />
ggives<br />
ives<br />
aan<br />
n ooptimal<br />
p t timal<br />
aand<br />
nd<br />
ppredictable<br />
redictable<br />
vvolume.<br />
olume.<br />
SSafe<br />
afe<br />
TTitanium<br />
itanium<br />
iis<br />
s a<br />
w<br />
well-examined, ell-examined,<br />
nnon-organic<br />
on-organic<br />
d ddental<br />
ental<br />
mmaterial<br />
aterial<br />
wwhich<br />
hich<br />
ddoes<br />
oes<br />
nnot<br />
ot<br />
iinvolve<br />
nvolve<br />
aany<br />
ny<br />
ppotential<br />
ot<br />
t ential<br />
rrisk<br />
isk<br />
oof<br />
f ccontamination.<br />
ontamination.<br />
Easy<br />
to<br />
use<br />
Stays<br />
in<br />
place,<br />
qquickly<br />
uickly<br />
fforms<br />
orms<br />
a<br />
ccoagulum<br />
oagulum<br />
w wwhen<br />
hen<br />
mmixed<br />
ixed<br />
wwith<br />
ith<br />
bblood.<br />
lood.<br />
For FFo<br />
or a distributor dist distributor di t near you, please please contact Tigran:<br />
info@tigran.se an.se or +46 40 693 92 70 770<br />
0<br />
35
36<br />
<strong>EDI</strong><br />
<strong>EDI</strong> News<br />
An interview with Professor Gerhard F. Riegl on success factors for dental offices<br />
Finding the right patients<br />
German dentists do not always have much of a grasp on marketing. Part of the reason is their professional statute that prohibits<br />
advertising, but also the usual tightrope walk between ethical principles and the need to make a profit, plus the reimbursement<br />
principle that applies to much of Germany’s healthcare system. <strong>EDI</strong> Journal spoke with Gerhard F. Riegl, Augsburg, about factors<br />
that determine the success of a dental office. Riegl is professor for marketing at the Faculty of Business at the Augsburg University<br />
of Applied Sciences and has been following the healthcare market and its protagonists for more than 25 years.<br />
Professor Riegl, you have followed up your book “Dental<br />
Office – Centre of Excellence” with a new book entitled<br />
“Success Factors for Dental Offices”. What is Riegl’s<br />
power marketing textbook all about?<br />
In a few words, it is about how knowledgeable dentists<br />
become successful managers – and this includes<br />
economic success. Good dentists should not complain<br />
about the mediocre performance of their colleagues<br />
nor wait for hopeless bunglers to disappear<br />
from the market. Rather, they should demonstrate<br />
that they themselves are ready to face and outperform<br />
any competition in terms of knowledge, organization<br />
and, especially, good customer service. This<br />
book is a book about ideas and the future and looks<br />
at dentists’ public image. This public image translates<br />
into goodwill, something that the field as a<br />
whole and all dentists can benefit from.<br />
The next step is to create a systematic master plan<br />
for the dental office. The objective has been to show<br />
how to find the right patients for one’s own office,<br />
today and tomorrow; how to successfully deal with<br />
these patients on an interpersonal level using the<br />
full range of professional tools available; and how to<br />
achieve patient loyalty in a natural, authentic way. We<br />
call this dental compliance coaching.<br />
You have surveyed patients at 11-year intervals and<br />
evaluated the results at your institute. How does this<br />
work, and where do you get your patients from?<br />
Our institute performs continuous systematic<br />
qualitative analyses for dental customers based on<br />
patient research. We do not enter the dentist’s<br />
premises, and we do not interfere with the day-today<br />
workings of the office; rather, we use professional<br />
methods to identify individual strengths and<br />
chances as well as areas that need improvement.<br />
This evaluation gives participating dentists benchmarking<br />
data, i.e. anonymous comparative data relative<br />
to other dental offices in the same region, to<br />
inform dentists’ decisions, to allow them either to<br />
determine that they are the best or that they can<br />
learn from the best. We are now doing this in eleven<br />
European countries, from Finland to Italy, and in<br />
seven languages, offering certificates for dentists<br />
and oral implantologists. Our analyses are building<br />
blocks for dental quality management, which will be<br />
mandatory from 2011 on and contribute to patientdriven<br />
value at the dental office.<br />
Your press release states that dentists enjoy an excellent<br />
reputation with their patients and that the metrics<br />
for quality and customer satisfaction indicate<br />
results between good and very good. What is it exactly<br />
that patients appreciate?<br />
Dentists have certain core competencies that<br />
define their image in popular perception. Factor<br />
number one is hygiene and cleanliness at the dental<br />
office, followed by a friendly dental team, treatment<br />
quality, respectful chairside manners, patient education<br />
and the overall office atmosphere.<br />
With so much light, can there be any shadow?<br />
Well, 47 per cent of all dental patients are not perfectly<br />
satisfied with their dentist’s overall performance,<br />
i.e. they do not always assign the highest marks.<br />
According to the results of our research, patients still<br />
criticize their dentists’ websites (if any), inappropriate<br />
presentation of the price-performance ratio of the<br />
different treatments, waiting times, patient education<br />
brochures, administrative issues, dental prevention,<br />
cooperation of their dentists with specialists and the<br />
availability and timing of appointments.<br />
A modern adage says that you don’t change hairdressers<br />
and you don’t change dentists. Can you prove<br />
that the second part of this adage is right?<br />
We have found that this used to be the case, because<br />
40 per cent of the older patients are still seeing their<br />
Professor<br />
Gerhard F. Riegl
<strong>EDI</strong><br />
<strong>EDI</strong> News<br />
very first dentist. By contrast, this is the case for only<br />
23 per cent of the less than 30-year-olds. On average,<br />
patients will have been with their current dentists for<br />
5.7 years. But the sustainable dentistry of the future<br />
will require even more sustainable patient loyalty.<br />
To what extent do patients consult the Internet or<br />
advertisements in choosing their dentists?<br />
We must differentiate between choosing the family<br />
dentist and choosing an implantological specialist.<br />
It is true that about 25 per cent of our patients use<br />
the Internet as one of their sources of information<br />
on dental topics or issues. But only 1 per cent of the<br />
patients resort to the Internet for finding their family<br />
dentist; for younger patients, the corresponding figure<br />
is 2 per cent. With oral implantologists, the share<br />
of patients who have visited the clinic’s website to<br />
familiarize themselves with the treatment provider is<br />
11 per cent. It was found that information about dental<br />
offices on the web is mostly used for self-assurance,<br />
for identification, for confirmation of one’s own<br />
views or to get information about things already<br />
experienced in vivo – not so much for selecting or<br />
comparing treatment providers.<br />
Patients increasingly use web portals to rate their dentists.<br />
A single negative rating can in principle stain your<br />
online reputation forever. In your book you state that<br />
new office strategies are required, including which you<br />
call “exit management”. What do you mean by that?<br />
Looking at the so-called patient rating portals on<br />
the web, we see a well-known fact re-materialize: In<br />
principle, any dentist can treat any patient, but no<br />
dentist can be the best dentist for all patients. If you<br />
want to be a dentist who is true to principle, authentic<br />
and credible in your patient relations, you will<br />
need to have the courage to say no if necessary and<br />
to refuse further involvement where the psychological<br />
circumstances offer no path towards a satisfactory<br />
solution. This means that patient relationships<br />
that are detrimental to the dentist and office – even<br />
though the dentist has done nothing wrong – will<br />
have to be terminated diplomatically before it is too<br />
late. We describe behavioural strategies for severing<br />
ties with patients while at the same time offering<br />
assistance in finding a dentist who is more likely to<br />
meet their specific needs.<br />
You have done some research on the referral behaviour<br />
of 3,000 family dentists and found that 29 per<br />
cent of all dentists now perform implant procedures<br />
themselves, while the rest collaborate with oral and<br />
maxillofacial surgeons or oral implantologists; yet<br />
family dentists state that only 22 per cent of their<br />
patients choose their oral implantologist themselves.<br />
37<br />
A-DE10001<br />
Aesculap ® Dental<br />
Bone Fixation<br />
Easy Handling – Excellent Results<br />
Bone Fixation Forceps:<br />
❚ Delicate and adaptable working tips<br />
❚ Universal application ➠ upper and lower jaw<br />
Screws:<br />
❚ Highest stability<br />
❚ Smallest possible screw head<br />
❚ Biocompatible<br />
❚ Easy removal of residues<br />
Aesculap AG | Am Aesculap-Platz | D-78532 Tuttlingen | Germany<br />
Phone +49 7461 95-2496 | Fax +49 7461 78980<br />
eMail dental@aesculap.de | www.aesculap-dental.com
38<br />
<strong>EDI</strong><br />
<strong>EDI</strong> News<br />
How does that fit in with the image of a new generation<br />
of patients who use the Internet to obtain the<br />
information they need?<br />
Never before have patients had access to as much<br />
health information as today, and this is as true of the<br />
dental field as of any other. However, this multifaceted,<br />
heterogeneous, sometimes contradictory data<br />
“haze” has not resulted in faster or more autonomous<br />
decisions; rather, a common result is information<br />
overload. Experience has shown that the more<br />
patients know, the more they appreciate that they<br />
know too little. Realization of this fact results in a<br />
desire for counsellors, pilots, navigators – preferably<br />
in a face-to-face setting. We are therefore witnessing<br />
a trend toward recommendation marketing and wordof-mouth<br />
recommendations. For dental patients, the<br />
family dentist continues to be their by far most<br />
important guide.<br />
Even though 90 per cent of all patients in Germany are<br />
covered by statutory health insurance and therefore<br />
should not have to worry too much about the cost of<br />
the treatment, more and more patients need advice<br />
on cost and financing issues. Is this because statutory<br />
health insurance does not cover all treatment options,<br />
or do today’s patients want to be informed of the cost<br />
of the treatment as a matter of principle?<br />
The dental field has played a pioneering role when<br />
it comes to patients accepting responsibility for their<br />
own health. Only 5 per cent of the patients covered<br />
by statutory health insurance believe that the standard<br />
treatment they have coverage for is sufficient<br />
for their specific case. Today, dental patients require<br />
much more information about cost and financing<br />
than about issues related to the treatment itself. For<br />
example, 21 per cent require more information about<br />
co-payments for implants, 18 per cent about co-payments<br />
for laboratory costs, 20 per cent about instal-<br />
Professor Gerhard F. Riegl<br />
ment options, 22 per cent require assurance that<br />
their restorations will be fabricated by a German laboratory<br />
led by a master dental technician and 49 per<br />
cent require detailed specification about what is<br />
reimbursable and what is not. A full 80 per cent<br />
wants to discuss cost openly and up front.<br />
The trend would indicate that the dentist-patient<br />
relationship is becoming more rational. However, this<br />
must not result in the dentist taking on the role of a<br />
salesperson. Dentists should be the patients’ consultants<br />
and trustees, not a mere provider of services.<br />
Confusing the patient role defined by the standards<br />
of social interactions with the customer role defined<br />
by the standards of market interactions is not permissible.<br />
Patients want their dentists to treat them<br />
better than any customer would be treated.<br />
So what do the results of this study tell us about who<br />
is the ideal dentist for most patients?<br />
Our patient surveys increasingly indicate that<br />
patients want dentists to understand and appreciate<br />
them, in an atmosphere of honesty, kindness and<br />
friendship. Patients want to get a sense of partnership<br />
and security when visiting their dentists. They<br />
want to feel safe. Dentists capable of creating this<br />
type of atmosphere relieve anxiety, build confidence<br />
and qualify for their intended role as benevolent<br />
patient guides. The ideal dentist does not specialize<br />
in implants, dental prevention or endodontics. The<br />
ideal dentist specializes in people and helps patients<br />
behave as they always wanted to behave when it<br />
comes to oral hygiene and dental health. Ultimately,<br />
the issue is whether the dentist is capable not only of<br />
performing good dental treatment, but also of soothing<br />
and calming the patient’s soul.<br />
Professor Riegl, thank you very much for this insightful<br />
interview. AWU<br />
Gerhard F. Riegl, Dr rer pol, is Professor for Marketing at the Augsburg University of Applied Sciences, Faculty of Business. He is<br />
a university teacher in the field of international market management and the founder and Scientific Director of the Health<br />
Care Management Institute in Augsburg. He has been conducting research on patients and referrers for 34 years, resulting in<br />
pioneering publications, e.g. the German-language monograph “The Dental Office as a Centre of Excellence”, on marketing<br />
activities by dentists, physicians and hospitals.<br />
As a trailblazer of dental marketing in Germany, Riegl has overseen more than 40,000 evaluations of dentists by patients<br />
treated at 700 different dental offices. He is held to be one of the leading health management trainers in Germany. Riegl<br />
is the author and publisher of the first major dental image and marketing study; he has authored more than 300 articles<br />
in other publications on health care marketing and conducts national and international research projects on dental and<br />
health care marketing. He works with chambers of dentists, professional associations and dental working groups in Germany<br />
on issues related to quality management, marketing and human-research management.The German newsmagazine Focus has<br />
described Riegl as one of the most eminent experts on hospital management and medical quality assurance.
40<br />
<strong>EDI</strong><br />
<strong>EDI</strong> News<br />
European Parliament: PIL on the web<br />
In the future, patients will find comprehensive and<br />
intelligible information about prescription drugs on<br />
the web. Advertising such drugs, on the other hand,<br />
will continue to be prohibited, according to the European<br />
Parliament in Strasbourg. As reported by the<br />
large German daily Frankfurter Allgemeine Zeitung,<br />
patients and their families are supposed to receive<br />
basic information about the benefits and risks of<br />
medicinal products, as well as on alternative therapies<br />
and preventive methods on the websites and<br />
web portals of the relevant national and European<br />
authorities. In addition, patient information leaflets<br />
(PIL) will have to be made available in all official EU<br />
languages. The industry should be prohibited from<br />
actively contacting patients and be restricted to<br />
responding to patient requests. Detailed information<br />
about the medicinal products they sell, including<br />
price or packaging information, can be published on<br />
manufacturers’ websites. However, this information<br />
must link to the official national and European health<br />
portals. To prevent companies from manipulating the<br />
information provided such that it effectively constitutes<br />
advertisement, all information is supposed<br />
to be examined and pre-authorized by the national<br />
authorities and the European Medicines Agency, headquartered<br />
in London.<br />
Source: European Parliament/<br />
Frankfurter Allgemeine Zeitung<br />
Europe-Ticker<br />
Czech Republic:<br />
Doctors threaten with exodus<br />
Czech hospitals have experienced a wave of physicians<br />
giving notice to leave their positions that may<br />
seriously threaten the country’s healthcare system<br />
and its patients. Hundreds of predominantly younger<br />
doctors have signed a declaration stating their intention<br />
to quit their jobs, responding to a call by the<br />
Czech Physicians’ Union that seeks to push through<br />
massive salary increases in this way. Under the motto<br />
of “Thank you, we’re leaving”, the physicians threaten<br />
to emigrate to Germany and other Western European<br />
countries, where their pay would be vastly better.<br />
These activities have already attracted a great<br />
deal of attention not only in the Czech Republic but<br />
also in other Central and Eastern European states,<br />
which also experience a mass exodus of local doctors<br />
and nurses. They are being courted by Western hospitals<br />
and healthcare institutions, which offer higher<br />
salaries and better work conditions. Thirty-two hospitals<br />
from Germany and one from Austria presented<br />
themselves at a job fair in Prague several weeks ago.<br />
The more than 5,000 Czech physicians who attended<br />
were offered monthly salaries of between €3,700<br />
and €4,500, which is roughly twice the currently prevailing<br />
salary level in the Czech healthcare system.<br />
Source: Various media<br />
European Parliament:<br />
New directive for treatment abroad<br />
Now even patients covered by national statutory<br />
health insurance can elect to be treated in hospitals<br />
or by specialists of their choice across the EU – at<br />
least if they would have to wait an unacceptably long<br />
time for the operation or if better specialist care for<br />
their specific disease is available in another member<br />
state. This, in short, is the content of a new EU directive<br />
agreed upon between the member states and<br />
the European Parliament following extensive and<br />
drawn-out negotiations. Patients seeking treatment<br />
in another member state will have to request prior
IDS COLOGNE<br />
March 22-26, 2011<br />
HALL 10.1<br />
BOOTH H050/J059<br />
CHIROPRO L<br />
ERGONOMICS<br />
REDEFINED<br />
With the 20:1 contra-angle of the Chiropro L system, you'll rediscover<br />
the meaning of the word ergonomics. This Swiss Made instrument<br />
has an internal irrigation system, unique in the world, which leaves<br />
the handling area completely free. The irrigant is directed at the base<br />
of the drill, running along it precisely. It offers perfect irrigation, especially<br />
for fitting implants using surgical guides. With the smallest head on<br />
the market and a very light weight, this instrument is as easy to handle<br />
as it is precise.<br />
Its LED lighting with two glass rods diffuses a natural white light and its<br />
power can be adjusted to suit your requirements. It is so comfortable,<br />
you could almost forget that the 20:1 contra-angle of the Chiropro L is<br />
driven by the MX-LED, the most powerful micromotor in the world.<br />
The unit also incorporates 7 of the leading implant systems on the market<br />
with their complete sequences.<br />
CHIROPRO L – increase your expectations<br />
Bien-Air Dental SA Länggasse 60 P.O. Box 2500 Bienne 6, Switzerland Phone +41 (0)32 344 64 64<br />
Fax +41 (0)32 344 64 91 dental@bienair.com www.bienair.com
42<br />
<strong>EDI</strong><br />
<strong>EDI</strong> News<br />
authorization from their health insurer; however, this<br />
authorization may not be arbitrarily refused. Moreover,<br />
they will need the financial means to advance<br />
the cost of treatment. Health insurance providers<br />
only have the obligation to reimburse patients for<br />
the cost incurred, and that only up to the amount<br />
customarily charged in the patient’s state of residence<br />
for comparable services. While this has little<br />
practical effect for patients in some countries, such<br />
as Germany, where reimbursement for treatment<br />
abroad has been the norm, in particular patients<br />
from Britain, Spain or the newer EU member states<br />
will benefit from the effects of this directive, which<br />
overrules existing restrictions on a national level.<br />
However, these liberal rulings do not cover medical<br />
services that are illegal in the patient’s member state<br />
of residence. For example, German women cannot<br />
expect to have pre-implantation diagnostics performed<br />
in Belgium and then receive reimbursement<br />
from their health insurer at home. You will find a<br />
detailed report on this directive in this issue of <strong>EDI</strong><br />
Journal.<br />
Source: Various media<br />
United Kingdom:<br />
A health reform that is visible from space<br />
The British conservative-liberal government has<br />
brought a comprehensive health reform on its way.<br />
Sir David Nicholson, chief executive of the National<br />
Health Service (NHS), has described the proposals by<br />
Health Secretary Andrew Lansley as the biggest<br />
change management program in the world – so<br />
large “that you can actually see it from space”. The<br />
reform intends to bring the public health system into<br />
closer alignment with the market economy. Up to<br />
now, the NHS has been funded entirely by the taxpayer.<br />
Of the enormous healthcare budgets, 60 per<br />
cent are spent on the salaries of the 1.7 million NHS<br />
employees and 20 per cent are spent on procuring<br />
medicinal products. The remainder of the money<br />
covers the cost of new medical facilities. However,<br />
facilities have been seriously underfunded for many<br />
decades. The current inefficiency is the problem Lans-<br />
Photo: Panthermedia/Alexander Zschach<br />
ley has set out to address. Patients, physicians and<br />
hospitals are to be given incentives to identify the<br />
most economical and most beneficial treatment.<br />
Patients will then be able to choose their treatment<br />
providers, general practitioners (GPs) and specialists<br />
can refer patients to competing private or stateowned<br />
surgical clinics for treatment, and private and<br />
state clinics can freely compete for both staff and<br />
patients. GPs and specialists will be given control<br />
over the largest chunk, £70 million, of an overall<br />
healthcare budget of £100 million a year. Physicians<br />
are to form joint committees to decide what medical<br />
provider they want to use to direct the treatment of<br />
their patients. The more skilful they prove to be in<br />
doing so, the more extensive will be the amount of<br />
state funds allotted to them. Sceptics fear that private<br />
surgical centres will practice cherry-picking, performing<br />
only lucrative routine operations, with state<br />
hospitals being stuck with expensive complicated<br />
surgery and treatments and becoming even less efficient<br />
than today. The government is hoping for closures<br />
and mergers among dental offices and hospitals;<br />
this, in their view, is one of the main points of<br />
the reform.<br />
Source: Various media<br />
European Commission: Consultations on<br />
professional qualifications initiated<br />
The European Commission has started the public<br />
consultation process on the Professional Qualifications<br />
Directive (2005/36/EC). These consultations<br />
offer participants an opportunity to submit their<br />
views on aspects of the directive they believe could<br />
be simplified and made more user-friendly. Comments<br />
are also solicited on how professionals working<br />
in the European Single Market can be better integrated,<br />
and on a European Professional Card.<br />
This directive forms the basis for professionals<br />
unleashing the full potential of the Single Market<br />
when looking for a new position or seeking to extend<br />
their professional activities in another member state.<br />
In 2012, the Commission will make a legislative proposal<br />
on modernizing this directive. Stakeholders were<br />
able to submit comments until 15 March 2011.<br />
The Professional Qualifications Directive covers<br />
800 professions regulated by the member states<br />
that can only be exercised by holders of certain professional<br />
qualifications. The qualifications of a number<br />
of healthcare professionals, including dentists,<br />
are covered by the automatic recognition mechanism,<br />
based on the harmonization of educational<br />
and training requirements throughout the EU.<br />
Source: Various media
10_ZES_006_LOC Ad_210mmx297mm_<strong>EDI</strong> Trim: 210mmx297mm 4-Color<br />
Dual retention, pivoting action reduces the wear<br />
and tear even on divergent implants.<br />
We pioneered self-aligning overdenture a� achments to combat<br />
the damage done by the improper seating of overdentures. Today,<br />
over 70 manufacturers have partnered with ZEST Anchors to<br />
customize our Locator pivoting technology abutments to be<br />
interface compatible with over 350 diff erent implant products.<br />
Patients the world over are enjoying an unprecedented quality of<br />
life, as they no longer have to worry about ill-fi � ing dentures. All<br />
in all, a compelling argument for you to consider incorporating the<br />
industry’s most comprehensive overdenture a� achment system<br />
into your practice, regardless of the implant system your prefer.<br />
Be sure to visit us at IDS, Hall 4.2, Booth #G77 and learn about the<br />
ZEST Locator A� achment System.<br />
For more information about the ZEST Locator Attachment System,<br />
please call +1-760-743-7744 or visit www.zestanchors.com.<br />
©2011 ZEST Anchors LLC. All rights reserved. ZEST ® and LOCATOR ® are registered trademarks of ZEST IP Holdings, LLC.<br />
Two-implant overdenture with<br />
ZEST Locator A� achment System<br />
compensating for divergent implants.<br />
10_ZES_006_LOC AD_210x297_<strong>EDI</strong>.indd 1 2/14/11 3:55:33 PM
44 <strong>EDI</strong><br />
European Law<br />
New EU Directive<br />
More rights for patients<br />
With its approval of the “Directive on the Application<br />
of Patients’ Rights in Cross-border Healthcare” in mid-<br />
January, the European Parliament has reached an<br />
important milestone on the road to more free movement<br />
and more freedom to render services. The core<br />
message: patients generally have the right to obtain<br />
health services in all member states without requiring<br />
prior authorization by their health insurer. In<br />
doing so, they have a right to reimbursement of<br />
their cost. This vote finally gives not only patients<br />
but also members of the healthcare professions as<br />
well as social security institutions legal clarity: in the<br />
future, member states must afford patients better<br />
support as they utilize cross-border healthcare services.<br />
More than a decade after the landmark decisions<br />
by the European Court of Justice (ECJ) in the<br />
Kohll and Decker cases, the principles embraced in<br />
these decisions have finally been codified and must<br />
now be implemented in national law by the member<br />
states.<br />
The principles of the Patient Rights Directive are<br />
not new. They can be directly derived from European<br />
Union contractual law, according to which limiting<br />
the free selection of doctors is not compatible with<br />
fundamental European rights. The ECJ has decided<br />
along these lines several times since 1998, and the<br />
same principles were embodied in the European<br />
Commission’s 2004 draft Directive on Services in the<br />
Internal Market. Nevertheless, EU citizens still repeatedly<br />
had to seek recourse from the Luxembourgbased<br />
court to assert their rights in their specific<br />
cases. Notable examples include that of Raymond<br />
Kohll, who was refused reimbursement for orthodontic<br />
treatment at a dentist in Trier, Germany by Luxembourg’s<br />
Caisse de Maladie. Or Nicolas Decker, a citizen<br />
of Luxembourg who won the right to reimbursement<br />
for the cost of glasses with corrective lenses<br />
made in Arlon, Belgium from his health insurance<br />
fund. Or Aikaterini Stamatelaki, who sued the Greek<br />
health insurer for the liberal professions, Organismos<br />
Asfaliseos Eleftheron Epangelmation, before the ECJ<br />
for reimbursement of the treatment cost incurred for<br />
her late husband at London Bridge Hospital, a private<br />
hospital in the United Kingdom. The proceedings<br />
dragged out for ten years before the ECJ finally ruled<br />
in favour of the plaintiff in 2007.<br />
Time and again, social security<br />
institutions and national health services<br />
have negated the freedom to perform services<br />
in the healthcare sector in the European internal<br />
market, even though the free movement of people,<br />
goods, services and capital is part of the European<br />
fundamental freedoms. Article 49 of the EG treaty<br />
prohibits restrictions on the provision of intra-community<br />
services. Restrictions are said to exist already<br />
if the provision of these services is made more difficult<br />
or less attractive or fraught with potential obstacles,<br />
which includes discriminatory practices in the<br />
general environment of the service in question.<br />
The ECJ has pointed out that it is not permissible<br />
for member states “to exclude the public health sector,<br />
as a sector of economic activity and from the<br />
point of view of freedom to provide services, from the<br />
application of the fundamental principle of freedom<br />
of movement”. The court has repeatedly stated that<br />
peculiarities surrounding the provision of specific<br />
services do not abridge the freedom to provide services<br />
and related principles. Nor does it matter how<br />
the healthcare system in the patient’s home country<br />
is organized.<br />
Reverse discrimination remains in place<br />
The directive now adopted obliges member states<br />
to refrain from requiring prior authorization before<br />
reimbursing patients for the cost incurred for healthcare<br />
services provided in another member state. If<br />
the cost of these services would have been paid<br />
for by the social security institutions in question, had<br />
they been provided in their own territory, patients<br />
must be reimbursed for them if the services were<br />
provided in another member state. Whether or not<br />
the patient’s home country adheres to the principle<br />
of providing benefits in kind is immaterial in this<br />
context. For patients covered by German statutory<br />
health insurance, this means that they can be treated<br />
in the same manner as patients covered by German<br />
private health insurance (which follows the<br />
reimbursement principle), even though they are<br />
(only) entitled to benefits in kind within Germany.<br />
Many observers have seen this as an example of<br />
reverse discrimination. However, reverse discrimina-
46 <strong>EDI</strong><br />
European Law<br />
tion is a non-topic within the context of European<br />
law. It is lawful for Germany to afford its residents<br />
covered by statutory health insurance fewer rights at<br />
home than abroad. A similar situation prevails with<br />
regard to physicians and dentists who are not accredited<br />
by the German health insurance system; their<br />
colleagues in member states outside Germany may<br />
legally treat patients covered by statutory health<br />
insurance without such authorization. It would be<br />
desirable for legislators to draw the right conclusions<br />
from the newly adopted directive. The extended<br />
options for applying the cost reimbursement principle<br />
provided by recent statutory health insurance<br />
legislation are a step in the right direction.<br />
Cost reimbursement only abroad<br />
In the future, patients must also be reimbursed for<br />
the cost of medicinal products prescribed and<br />
obtained in the context of healthcare-related services.<br />
Restrictions are only admissible if and to the<br />
extent that the utilization of cross-border healthcare<br />
services would threaten the financial balance of the<br />
social security systems in the member states. For<br />
example, the freedom to provide services could be<br />
restricted with regard to inpatient care if increased<br />
patient mobility would jeopardize the objective of<br />
providing a well-balanced supply of high-quality hospital<br />
treatment alternatives in a specific member<br />
state. Unfortunately, it must be expected that health<br />
insurers will invoke this very provision to hinder the<br />
increasing mobility of patients.<br />
The Ministers of Health had already acknowledged<br />
years ago that the EU is home to shared values and<br />
principles with regard to healthcare. These include<br />
universality, access to high-quality care, distribution<br />
fairness/equality and solidarity. For this reason, the<br />
member states should ensure that these shared values<br />
and principles are also applied to patients and<br />
citizens from other member states. Regardless of<br />
where they are from and what specific health insurance<br />
arrangement they have – all patients must be<br />
treated alike. However, this does not imply any right<br />
to preferential treatment. On the contrary: The freedom<br />
to provide services may be limited if an increase<br />
in demand results in longer waiting times or capacity<br />
bottlenecks for residents.<br />
Quality and safety<br />
The Patient Rights Directive requires member states<br />
to make “systematic and continuous efforts ... to<br />
ensure that quality and safety standards are im -<br />
proved”. The original plan had been to define standards<br />
at a European level, but the attempt failed<br />
because the EU has no authority to regulate the<br />
healthcare sector.<br />
EU member states will have to establish contact<br />
points offering information on healthcare providers.<br />
On request, these contact points will also provide<br />
information on a specific provider’s right to provide<br />
services or any restrictions on its practice. They are<br />
also tasked to educate the public on patients’<br />
rights, complaint procedures and mechanisms for<br />
seeking remedies, for example on mediation mechanisms.<br />
Member states must also ensure that the<br />
national contact points consult with patient organizations,<br />
healthcare insurers and healthcare providers<br />
as appropriate.<br />
The Patient Rights Directive includes another<br />
important innovation: member states should ensure<br />
that patients from other member states receive<br />
information on safety and quality standards enforced<br />
on its territory. On request, physicians and dentists<br />
should provide patients with information on specific<br />
aspects of the healthcare services they offer and on<br />
the treatment options.<br />
Outlook<br />
At this point, it is still the member states, not the<br />
European Commission, that are competent to regulate<br />
health care. Nevertheless, it is obvious that the<br />
European institutions are beginning to extend the<br />
range of their activities in this field. This implies a<br />
threat of additional regulation and standardization.<br />
For this reason, the consultation process now being<br />
conducted by the EU Commission on the 2006 Directive<br />
on Professional Qualifications should be watched<br />
closely. This directive defines professional minimum<br />
standards e.g. for exercising the profession of dentist<br />
within the EU. It is widely feared that the standards<br />
will be relaxed in order to promote the mobility of<br />
healthcare service providers.<br />
Politicians in EU member states are tasked to find<br />
the right balance between the desirable liberalization<br />
within the healthcare sector and the necessary<br />
regulation benefiting the interests of healthcare<br />
providers and patients alike, a balance that considers<br />
the interests of all stakeholders. The new Patient<br />
Rights Directive provides some important stimuli in<br />
the right direction.<br />
Peter Knüpper, attorney at law<br />
Joint practice of Dr Rehborn<br />
Munich<br />
Germany
48 <strong>EDI</strong><br />
European Law<br />
On the legality of prior authorization<br />
requirements for outpatient treatment<br />
requiring the use of major medical<br />
devices in a different member state<br />
In its decision dated 5 October 2010 (C-512/08), the<br />
European Court of Justice in Luxemburg (ECJ) was<br />
called upon to decide whether statutory health<br />
insurers may require authorization prior to treatment<br />
using major medical equipment in another<br />
member state.<br />
The case<br />
For a change, the court was not called upon this<br />
time to decide on an action brought by a patient<br />
against a health insurer with regard to the reimbursement<br />
of treatment costs. Rather, the issue at<br />
hand was a letter of formal notice sent to the French<br />
Republic by the European Commission. Its specific<br />
complaint was that French national regulations<br />
require prior authorization by the statutory health<br />
insurer as a condition for reimbursement of the<br />
cost of medical treatment in another member state<br />
requiring the use of major medical equipment. The<br />
relevant article of the Public Health Code provides an<br />
exhaustive enumeration of the major medical equipment<br />
affected, namely PET scanners, magnetic resonance<br />
imaging apparatus, medical scanners, hyperbaric<br />
chambers and cyclotrons for medical use.<br />
According to the French rules, prior authorization is<br />
needed only for proposed treatments, not for cases<br />
where the need for the use of this equipment is<br />
unexpected. This means that the prior authorization<br />
requirement would not apply to an unforeseen treatment<br />
for which a need arises while the insured person<br />
is temporarily staying in another member state.<br />
According to the French Social Security Code,<br />
authorization may be refused only if one of the following<br />
conditions applies: (1) the proposed treatment<br />
is not one of those in respect of which the French<br />
rules provide for responsibility for its payment; (2)<br />
treatment that is identical or equally effective can be<br />
obtained in good time in France, taking into account<br />
the patient’s condition and the likely development of<br />
the patient’s illness. Social security institutions are<br />
advised that to refuse authorization, it is not sufficient<br />
to point out the existence of treatment alterna-<br />
tives in general; rather, any such refusal must be<br />
based on facts, for example by providing patients<br />
with a list of establishments capable of administering<br />
the treatment needed within the period required.<br />
Healthcare institutions in France are not free to<br />
purchase major medical equipment for treating<br />
patients under the statutory healthcare system as<br />
they see fit; rather, they are themselves subject to<br />
authorization by the competent regional healthcare<br />
authority to deploy such equipment. This rule<br />
intends to ensure an adequate regional balance in<br />
purchase planning and deployment.<br />
ECJ decision<br />
In earlier decisions, the ECJ had ruled that national<br />
health insurers are not allowed to make the reimbursement<br />
of costs incurred for outpatient treatment<br />
in other EU member states contingent on prior<br />
authorization. The prior authorization requirement,<br />
however, was deemed acceptable in the case of<br />
inpatient treatment, but only with regard to planned<br />
treatment; certainly not in the event of unforeseeable<br />
hospital treatment, e.g. as a result of an accident.<br />
The ECJ had previously justified its stance on<br />
treating outpatient and inpatient services differently<br />
by recognizing that more extensive planning needs<br />
and practical constraints govern the provision of in -<br />
patient services. Such planning requirements relate,<br />
on the one hand, to ensuring sufficient and permanent<br />
access to a balanced range of high-quality treatment<br />
and, on the other, to the wish to control costs.<br />
The court has now applied this line of arguments<br />
to outpatient treatment with the major medical<br />
equipment as mentioned, by elaborating that a<br />
prior authorization requirement may be acceptable<br />
in order to avoid, as far as possible, any waste of<br />
financial, technical and human resources. However,<br />
this authorization requirement must apply to areas<br />
that are subject to a general planning policy in the<br />
respective member state, as is the case in France<br />
with regard to major medical equipment. This planning<br />
policy must have as its objective to ensure,
<strong>EDI</strong> 49<br />
European Law<br />
throughout the national territory, a rationalized, stable,<br />
balanced and accessible supply of up-to-date<br />
treatment, and also to avoid wastefulness wherever<br />
possible. Only when this is the case can the same<br />
line of arguments be used to require authorization<br />
prior to treatment using such equipment in another<br />
member state.<br />
The European Commission had argued that several<br />
circumstances permitted the inference that to do<br />
away with the prior authorization requirement in the<br />
sphere of treatment involving the use of major medical<br />
equipment would not lead to a huge exodus of<br />
insured persons from the French system to other<br />
member states and would not endanger the financial<br />
balance of the national social security system.<br />
The circumstances cited by the Commission included<br />
linguistic and geographic factors, the lack of information<br />
about the nature of treatment available in other<br />
member states, as well as necessary travel expenses.<br />
While the decision does not show that France actually<br />
refuted this argument, the ECJ in its decision simply<br />
assumes that abolishment of the prior authorization<br />
requirement could lead to under-use of the major<br />
medical equipment installed. This in turn might result<br />
in a disproportionate burden on the affected member<br />
state’s social security budget. The ECJ did not<br />
offer any more concrete guidance. It would thereby<br />
appear that the ECJ has clearly lowered the ribbon<br />
for member states wanting to institute such a prior<br />
authorization requirement by accepting as arguments<br />
general apprehensions without any concrete<br />
foundation.<br />
The only reservation cited by the ECJ relative to<br />
prior authorization requirements for outpatient<br />
treatment with major medical appliances is the current<br />
status of EU law. This reservation may have been<br />
introduced with a view to the impending Patients’<br />
Rights Directive, adopted by the EU Parliament in<br />
January 2011. From 2013 on, there will be uniform<br />
rules governing medical treatment in EU member<br />
states, which may also affect the situation concerning<br />
prior authorization requirements for treatment<br />
requiring the use of major medical equipment. The<br />
Council’s decision is still pending, meaning that the<br />
wording of the directive will probably not be finalized<br />
before later in the year.<br />
Solicitor Dr Berit Jaeger<br />
Ratajczak & Partners<br />
Berlin · Cologne · Essen · Freiburg ·<br />
Meissen · Munich · Sindelfingen<br />
Posener Straße 1, 71065 Sindelfingen<br />
Germany<br />
www.RPmed.de<br />
Û BY MECTRON<br />
CRESTAL SINUS<br />
LIFT – SAFE,<br />
SIMPLE AND FAST!<br />
Û SINUS PHYSIOLIFT® – MICROMETRIC<br />
DETACHMENT OF THE SCHNEIDERIAN MEMBRANE<br />
THANKS TO THE HYDRODYNAMIC PRESSURE<br />
mectron s.p.a., via Loreto 15/A, 16042 Carasco (Ge)<br />
Italia, tel +39 0185 35361, fax +39 0185 351374<br />
www.mectron.com, mectron@mectron.com
50 <strong>EDI</strong><br />
Clinical Science<br />
An implant-based treatment option for periodontally reduced dentitions<br />
A new therapeutic approach<br />
Dr Georg Bayer, Dr Frank Kistler, Dr Steffen Kistler, Stefan Adler, all Landsberg am Lech,<br />
and PD Dr Jörg Neugebauer, Landsberg am Lech and Cologne/Germany<br />
In recent years, the treatment of periodontal disease has seen developments that have resulted in various options<br />
for preserving the masticatory and phonetic function of compromised patients [1,6,12,13]. Patients can now select from<br />
a range of treatment options. Some patients are known for a critical attitude toward drug treatment, especially when<br />
it comes to systemic antibiotics, being afraid of adverse reactions and risks such as allergic reactions or antibiotic<br />
resistance. Furthermore, critical reports have been published notably with regard to the clinical results obtained in<br />
heavy smokers [2,6].<br />
Photodynamic therapy<br />
Topical treatments can only be performed in patients<br />
willing to return periodically for recall visits and continuous<br />
treatment to avoid recurrence of the disease<br />
with progression of the vertical bone loss. Photody-<br />
Fig. 1 Immediate implant insertion following extraction of the<br />
distalmost natural abutments above the mental foramen.<br />
Fig. 3 Parallelized impression posts used for the fabrication of<br />
the screw-retained provisional restoration.<br />
namic therapy has yielded promising results in this<br />
context by effectively reducing the number of<br />
pathogens in the absence of systemic side effects [8].<br />
If there is a need for a prosthetic restoration, however,<br />
Fig. 2 Connection of the angled abutments to the distal<br />
implants to adjust the path of insertion of the restoration.<br />
Fig. 4 The long-term resin provisional delivered at the end of<br />
the surgical treatment.
<strong>EDI</strong> 51<br />
Clinical Science<br />
the question arises how this treatment should be<br />
continued. In the presence of multiple tooth loss or<br />
with natural distal abutments missing, conventional<br />
prosthetic restorations can only be implemented in<br />
the form of removable dentures. Especially patients<br />
in their forties, however, are unlikely to accept dental<br />
treatment of this type. Due to the extensive social<br />
life of this age group, they will rarely content themselves<br />
with a removable denture [14,15]. On the other<br />
hand, very extensive treatment steps may be<br />
required for consecutive implant placement to substitute<br />
for teeth that can no longer be preserved [7].<br />
For one thing, the distribution of edentulous areas is<br />
often unfavourable, such that a relatively large number<br />
of implants will be required. For another, the<br />
need for repeated prosthetic restorations with consecutive<br />
implant placement may become quite<br />
expensive. One treatment option, therefore, is to<br />
remove teeth that are no longer worth preserving,<br />
even though they could theoretically be preserved,<br />
in favour of a full-arch implant-supported rehabilitation<br />
[4,5].<br />
Angulated implant abutments allow the implementation<br />
of fixed restorations supported by fewer<br />
implants, namely four implants in the mandible or<br />
six in the maxilla [9]. This is precisely where photodynamic<br />
therapy comes in, offering ways of eliminating<br />
all pathogens from the infected tissue by topical<br />
decontamination during immediate implant placement<br />
[11]. This will ensure that success, and hence a<br />
good prognosis of immediate implant placement,<br />
can be achieved. Given most patients’ reluctance to<br />
accept a removable denture, it is recommended to<br />
combine the immediate placement of implants with<br />
an immediate restoration [3]. Angulated insertion of<br />
implants will allow for the establishment of wide<br />
anteroposterior support, which is important for<br />
achieving primary implant stability and for splinting<br />
them [10]. For immediate restoration, a temporary<br />
resin structure is fabricated based on a reduced setup<br />
extending to the second premolars directly after<br />
the implants have been placed.<br />
The final prosthesis is delivered after osseointegration<br />
of the implants. As the patient will be wearing a<br />
fixed restoration by that time, this final rehabilitation<br />
can be implemented with due consideration being<br />
given to all relevant laboratory and restorative<br />
aspects. Furthermore, the temporary restoration also<br />
offers an opportunity to increase the vertical dimension<br />
of occlusion to compensate for any decrease<br />
that may already have occurred in the area of the<br />
support zone due to the presence of periodontal disease<br />
and the absence of teeth, thus utilizing the<br />
period of osseointegration to adjust a new vertical<br />
dimension secured by stable implant anchorage.<br />
Dental Instruments<br />
Implantology Instruments<br />
Oralsurgery Instruments<br />
Silicone Materials<br />
Xpect more at IDS<br />
hall 10.2 booth L 29<br />
Bodenseeallee 14-16<br />
78333 Stockach / Germany<br />
Tel.: +49 7771 64999-0<br />
Fax: +49 7771 64999-50<br />
www.kohler-medizintechnik.de
52 <strong>EDI</strong><br />
Clinical Science<br />
Fig. 6 Radiological control prior to definitive impression by<br />
the referring dentist.<br />
Options of prosthetic restoration<br />
Depending on the patient’s expectations, a spectrum<br />
of very different restorative options may be available,<br />
ranging from relatively simple designs including a<br />
non-precious metal framework to which a ceramic<br />
veneer is fused, all the way to sophisticated designs<br />
including zirconia restorations with custom veneering.<br />
Minimizing the number of implants for such<br />
rehabilitations in periodontally compromised patients<br />
will reduce the known risk of peri-implant disease or<br />
even implant loss, as fewer implants that might get<br />
affected will be present in the first place [16]. As the<br />
initial restoration following tooth extraction will be<br />
immediately delivered by the surgeon, the referring<br />
practitioner will be able to plan and deliver the final<br />
rehabilitation without time constraints. In this way it<br />
is possible to meet the expectations of patients who<br />
express a desire for fixed rehabilitation despite the<br />
presence of residual teeth likely to be lost in the<br />
foreseeable future. Care should be taken, however, to<br />
deliver an initial restoration that will offer a relatively<br />
good chewing comfort, thus constituting an<br />
Fig. 7 Lingual view following delivery of the definitive<br />
restoration by the referring dentist.<br />
improvement on the previously reduced and periodontally<br />
compromised dentition. With regard to the<br />
final prosthetic rehabilitation, an exact analysis of individual<br />
needs is required such that maximum patient<br />
satisfaction will be ensured at the end of treatment.<br />
As a rule, this goal is perfectly within reach, given the<br />
absence of any time constraints.<br />
A list of references can be found at www.teamwork-media.de<br />
Contact address<br />
PD Dr Jörg Neugebauer<br />
Zahnärztliche Gemeinschaftspraxis<br />
Dres. Bayer, Kistler, Elbertzhagen und Kollegen<br />
Von-Kühlmann-Str. 1<br />
86899 Landsberg am Lech<br />
Germany<br />
Phone: +49 8191 947666-0<br />
neugebauer@implantate-landsberg.de<br />
www.implantate-landsberg.de<br />
Fig. 5<br />
Healed abutments<br />
being checked for<br />
successful osseo -<br />
integration after<br />
three months.
ROXOLID ®<br />
THE NEW “DNA” OF IMPLANT MATERIALS<br />
ROXOLID ® – Exclusively designed to meet the needs of dental implantologists.<br />
Roxolid ® offers Confidence when placing small diameter implants Flexibility of<br />
having more treatment options Designed to increase patients’ acceptance of implant treatment<br />
Straumann ® SLActive<br />
... over 1 million implants sold!<br />
Please contact us at +41 (0)61 965 11 11. More information on www.straumann.com<br />
AD_210x276_Roxolid.indd 1 04.11.10 13:16
54 <strong>EDI</strong><br />
Clinical Science<br />
Comparison of the bone response to dental implants with threaded<br />
and non-threaded necks<br />
Marginal bone loss around<br />
endosseous implants<br />
Vitomir S. Konstantinovic, DDS, MD, MSci, PhD, Belgrade/Serbia<br />
The long-term survival of endosseous dental implants depends on the preservation of bone support. Bone loss may be<br />
reduced by the retention grooves (microthreads) or by the polished implant neck surface. The aim of this paper was to<br />
critically reconsider recently published papers on marginal bone loss around endosseous implants, using a MEDLINE search<br />
that examined treatment outcomes after insertion of dental implants with threaded and non-threaded neck designs.<br />
The long-term survival of endosseous dental im plants<br />
depends on the preservation of bone support. A loss<br />
of crestal bone compromises the treatment outcome<br />
and the patient’s oral health. In integrated crestal<br />
implants, the breakdown of the implant-tissue interface<br />
begins in the crestal region, i.e. below the mucosal<br />
penetration area. It has been reported that changes<br />
in the crestal marginal bone level occur during the<br />
early phase of healing [1], as an adaptation of the periimplant<br />
bone to occlusal loads [2] or as a long-term<br />
response to altered trajectories within the bone as a<br />
response to the increased function [3]. Moreover,<br />
implant designs and surface characteristics affect the<br />
rate of early bone loss. It has been suggested that<br />
bone loss may be reduced by retention grooves<br />
(microthreads) at the implant neck or by a polished<br />
implant neck surface, which prevents penetration of<br />
the mucosa and subsequent peri-implantitis [15].<br />
The aim of this paper was to critically reconsider<br />
recently published papers on marginal bone loss,<br />
bone gain and other clinical outcomes around<br />
endosseous implants. Our interest was to analyze the<br />
studies in which dental implants with threaded and<br />
non-threaded necks were compared. Furthermore,<br />
our clinical cases, their outcomes and follow-up<br />
results are indicative as a contribution to the discussion<br />
about the impact of the dental implant neck<br />
design on bone response. We have decided to include<br />
case histories as the second part of this paper in<br />
order to highlight the need for further research.<br />
Study design<br />
A search of the literature was performed to identify<br />
studies that analyzed marginal bone loss, bone gain<br />
and clinical outcomes following the insertion of<br />
endosseous dental implants. Inclusion criteria were:<br />
• human studies with comparison of survival of<br />
dental implants with threaded and non-threaded<br />
neck design,<br />
• studies with measurements of the marginal bone<br />
loss,<br />
• studies of bone gain following dental implant<br />
placement,<br />
• studies with follow-up of implant stability,<br />
• studies with respectable methodological quality<br />
(RCT, cohort studies).<br />
Articles reporting case results without controls were<br />
excluded. Clinical cases treated by us using dental<br />
implants with threaded and non-threaded neck<br />
design were included as supplementary data information.<br />
It also intends to highlight the need for<br />
future research through carefully planned large and<br />
long-term comparative studies.<br />
A MEDLINE search was performed to identify articles<br />
published in the preceding five years, examining<br />
treatment outcomes after insertion of dental im -<br />
plants with threaded and non-threaded neck designs.<br />
Four articles met the inclusion criteria and were<br />
included in this report (Tab. 1 to 3).<br />
Interventions<br />
Dental implants with different neck designs were<br />
compared and described as follows:<br />
Bratu (2009)<br />
A prospective study of 46 patients with indications<br />
for two adjacent implants in the posterior mandible.
Tab. 1<br />
MEDLINE<br />
search<br />
summary.<br />
Tab. 2<br />
Comparative<br />
studies evaluating<br />
endosseous<br />
implant designs<br />
with or without<br />
microthreaded<br />
necks.<br />
Tab. 3<br />
Evaluation of<br />
articles comparing<br />
endosseous<br />
implant designs<br />
with and without<br />
micro -<br />
threaded necks.<br />
Terms Hits Reviewed<br />
Search dental implants OR dental implantation, endosseous [MeSH] 19.455<br />
Search (dental implants OR dental implantation, endosseous [MeSH]) AND<br />
surface properties AND comparative study, limits ENGLISH, human, literature<br />
containing abstracts<br />
319 4<br />
Bibliographies from existing literature 0 0<br />
Total primary articles reviewed 4<br />
Author<br />
(year)<br />
Bratu<br />
(2009)<br />
Nicke nig<br />
(2009)<br />
Piao<br />
(2009)<br />
Shin<br />
(2006)<br />
Study<br />
design Population<br />
Pro -<br />
spective<br />
cohort<br />
N = 46<br />
female: NR<br />
age: 23-65<br />
years<br />
RCT N = 34;<br />
Ni = 133<br />
female: NR<br />
age: 45.2 (25-<br />
55) years<br />
RCT N = 54;<br />
Ni = 135<br />
female: 39%<br />
age: 36-78<br />
years<br />
RCT N = 68<br />
female: NR<br />
age: NR<br />
Diagnostic<br />
characteristics<br />
Indication for placement<br />
of two neighbouring<br />
implants in<br />
the same quadrant of<br />
the posterior<br />
mandible<br />
Bilaterally edentulous<br />
posterior mandibles<br />
with indication for<br />
implant placement<br />
Indication for implant<br />
placement<br />
Indication for implant<br />
placement<br />
Implant placement<br />
Rough<br />
surface,<br />
with<br />
micro -<br />
threads<br />
N = 46;<br />
Ni = 46<br />
N = 34;<br />
Ni = 70<br />
N = 21;<br />
Ni = 45<br />
N = NR;<br />
Ni = 38<br />
Rough<br />
surface,<br />
no<br />
micro -<br />
threads<br />
N = 17;<br />
Ni = 45<br />
N = NR;<br />
Ni = 34<br />
Machi ned<br />
surface,<br />
no<br />
micro -<br />
threads<br />
N = 46;<br />
Ni = 46<br />
N = 34;<br />
Ni = 63<br />
N = 16;<br />
Ni = 45<br />
N = NR;<br />
Ni = 35<br />
<strong>EDI</strong> 55<br />
Clinical Science<br />
Follow-up<br />
(%) LoE*<br />
12 months:<br />
96%<br />
Median 1.9<br />
(1.9-2.1)<br />
years: NR†<br />
12 months:<br />
NR†<br />
12 months:<br />
NR†<br />
N = Number of subjects; Ni = Number of implants; * The Level of Evidence (LoE) is based on study design and methods (very<br />
high, high, moderate or poor); † NR (not reported) = follow-up rate either not reported or precise follow-up rate could not be<br />
determined since the initial number of eligible patients or the number lost to follow-up were not provided<br />
Study design and methods Bratu (2009) Nickenig (2009) Piao (2009) Shin (2006)<br />
1. Type of study design Prospective<br />
cohort<br />
RCT† RCT RCT<br />
2. Statement of concealed allocation* N/A Yes Yes No<br />
3. Intention to treat* N/A No No No<br />
4. Independent or blind assessment No No No No<br />
5. Complete follow-up of >_ 85% Yes Yes Yes Yes<br />
6. Adequate sample size Yes Yes Yes Yes<br />
7. Controlling for possible confounding Yes Yes Yes Yes<br />
Level of Evidence High High High Moderate<br />
† RCT – randomized controlled trial; * Applies to randomized controlled trials only; N/A – not available<br />
High<br />
High<br />
High<br />
Mod -<br />
e rate
56 <strong>EDI</strong><br />
Clinical Science<br />
All patients received (a) one implant with a 1-mm polished<br />
neck and no retention grooves, and (b) one<br />
implant with a roughened surface and microthreads<br />
at the neck. Other implant characteristics (taper, titanium<br />
alloy with moderately rough surface) were the<br />
same for both implants.<br />
Nickenig (2009)<br />
In a split-mouth study, 34 patients with bilaterally<br />
edentulous posterior regions in the mandible randomly<br />
received implants with a machined neck on<br />
one side and implants with a microthreaded neck on<br />
the contralateral side. Subjects were followed for two<br />
years under functional load.<br />
Piao (2009)<br />
54 patients randomly received implants with either<br />
(a) a rough surface (TiUnite Brånemark MK III), (b) a<br />
machined surface in the coronal part and rough surface<br />
in the apical part of the fixture (Restore) or (c) a<br />
rough surface with microthreads (Hexplant Oneplant).<br />
Subjects were followed for one year after<br />
implant loading.<br />
Shin (2006)<br />
In a prospective study, 68 patients were randomized to<br />
receive implants with either (a) a machined neck (Ankylos),<br />
(b) a rough-surfaced neck (Stage 1) or (c) a roughsurfaced<br />
neck with microthreads (Oneplant). Subjects<br />
were followed for one year after implant loading.<br />
Implant survival<br />
Implant survival was 100 per cent for both polishedneck<br />
and microthreaded-neck implants after one<br />
year of function. [Bratu]<br />
In the other three studies the authors have not<br />
specified the rate of implant survival, but neither<br />
implant loss was reported at the final examination.<br />
Marginal bone loss (Figs. 1 and 2)<br />
The marginal bone loss as measured on digitized<br />
panoramic radiographs was significantly greater for<br />
polished-neck compared to microthreaded-neck<br />
implants on the day of prosthesis cementation, four<br />
months after implant placement (0.77 ± 0.46 mm<br />
vs. 0.21 ± 0.19 mm, p < 0.05), after six months (1.20 ±<br />
0.44 mm vs. 0.56 ± 0.23 mm, p < 0.05), and after<br />
twelve months in function (1.47 ± 0.40 mm vs. 0.69 ±<br />
0.25 mm, p < 0.05). [Bratu]<br />
The marginal bone loss as measured on digitized<br />
panoramic radiographs was significantly greater for<br />
machined-surface (mean, 1.1 mm; range, 0-3 mm) compared<br />
to rough-surface, microthreaded (mean, 0.5 mm;<br />
range, 0-2.1 mm) implants (p < 0.001) after a median<br />
of 1.9 years in function. [Nickenig]<br />
Marginal bone loss/gain<br />
2.5<br />
2<br />
1.5<br />
1<br />
0.5<br />
0<br />
-0.5<br />
-1<br />
-1.5<br />
-2<br />
Rough surface, with microthreads Rough surface, NO microthreads<br />
Machined-surface, NO microthreads<br />
12 months, n=46<br />
[Bratu]<br />
23 months, n=34<br />
[Nickenig]<br />
p
���� ����<br />
����� ���<br />
����� ���� ��������<br />
������ ������� �� �����<br />
��� ���� & ����� �<br />
������ ����� �������������<br />
����� �������� ���� �� ���� ������ ����� � �������<br />
������������ ������ ����������� ����� �������� ������<br />
�����<br />
���� ��������� ������ ��� �� ��������� ���� ��� ���<br />
���� & ����� � ����������� ������� ��� ���������<br />
������������ �� ��� ���������� ���� �� ���������<br />
������ ��������� ���������� �������� ��� ��� ���<br />
����� ��� ��������� ����� ������������ ����������<br />
��� ������� ��� ������� ������������� ������� ��<br />
��� ����������� �� ���������<br />
���������� � ������������ � ��������� ����� ������<br />
������� ������� ���� & �����<br />
������������� ���� �<br />
����� ������ � �������<br />
���� ����� � �� �� � � �� � � ��<br />
��� ����� � �� �� � � �� � � ��<br />
�����������������������<br />
������ ������������������������
58 <strong>EDI</strong><br />
Clinical Science<br />
Since multiple implants in the same subject are<br />
not statistically independent, either one implant<br />
should be chosen per patient or statistical analysis<br />
should account for multiple implants per patient.<br />
This was not the case in three studies where multiple<br />
implants were placed in the same patient.<br />
Discussion<br />
Since the early 90s, many clinical studies have<br />
been conducted on the effect of the microthread<br />
on crestal bone loss. This was a variation on the<br />
guidelines for implant design derived from the<br />
Brånemark system – a smooth machined surface was<br />
regarded as an effective design to prevent plaque<br />
accumulation.<br />
However, it was soon realized that this machined<br />
neck is not an effective design for the distribution of<br />
occlusal forces. Hansson reported in 1999 that if perfect<br />
interlocking between the implant and the bone<br />
occurs and bone prevails apically to the neck region,<br />
interfacial shear stresses in the neck will not depend<br />
on the way in which interlocking was made. Hansson<br />
and others found that a small thread had the additional<br />
advantage of increasing the axial stiffness of<br />
the implant and brought more reduction in the peak<br />
interfacial shear stress on the cortical bone. Lee et al.<br />
suggested in 2007 that microthreads on the coronal<br />
portion of the fixture might have an effect in maintaining<br />
the marginal bone loss against loading,<br />
based on a 3-year prospective study.<br />
All authors of the studies reviewed reported the<br />
least amount of bone loss in the group for the roughsurfaced<br />
microthreaded neck implants after one<br />
year of functional loading, whereas the group with<br />
machined-neck designs showed the greatest amount<br />
of bone loss.<br />
It still appears difficult to draw clear conclusions<br />
on the impact of macrodesign of the implants when<br />
considering microthreads on the neck.<br />
When bone loss rate was compared, Shin (2006)<br />
and Bratu (2009) reported somewhat different<br />
results. Shin noticed that most bone loss occurred<br />
early in the healing period, which agrees with the<br />
results of other authors. However, the implants with<br />
machined necks showed significant bone loss during<br />
every interval of the follow-up, which could be interpreted<br />
as progressive bone loss. Bratu noticed that<br />
the rate of bone loss differed between implants with<br />
machined necks and implants with microthreaded<br />
necks only during the first six months; furthermore,<br />
he speculated that the surface roughness determines<br />
the amount of early bone loss, not the<br />
macrodesign. Finally, all authors are cautious in their<br />
interpretation of the results and the exact impact of<br />
Marginal bone loss (mm)<br />
1.6<br />
1.4<br />
1.2<br />
1.0<br />
0.8<br />
0.6<br />
0.4<br />
0.2<br />
0.0<br />
p
CAMLOG<br />
AT THE IDS:<br />
HALL 11.3,<br />
BOOTH<br />
NO. A10-B19<br />
VARIO SR SCREW-RETAINED<br />
COMPONENTS FOR<br />
MORE POSSIBILITIES<br />
For occlusally screw-retained crown and bridge restorations. Proven CAMLOG handling.<br />
Improve safety, save time thanks to special aligning tool. CAMLOG offers more.<br />
Further information: www.camlog.com
60 <strong>EDI</strong><br />
Clinical Science<br />
Fig. 3a Implant with threaded neck design before insertion.<br />
Fig. 4a OPG – Implants inserted at sites 14, 34 and 44 with<br />
threaded neck design. Implants at sites 15, 17, 35, 36, 37, 45, 46<br />
and 47 with polished surfaces.<br />
Case 1 – female, 56 years old<br />
The patient received a combination of machined and<br />
microthreaded implants (Figs. 3a and b). In Figure 4b,<br />
in comparison to Figure 4a, it is clearly visible that a<br />
larger amount of the marginal bone is lost around<br />
the dental implants with machined surface.<br />
Unlike crestal implants, basal implants (BOI, TOI,<br />
Diskimplant) do not cause progressive crestal bone<br />
loss over time along their vertical portions, and they<br />
do not trigger any crater-shaped areas of collapsed<br />
bone. An example of progressive bone loss around<br />
crestal implants is shown in Figure 5. There have been<br />
isolated reports that restorations based on basal<br />
implants not only arrest the bone loss brought about<br />
by periodontal disease but can also generate new<br />
bone tissue [9,10]. Over the course of the development<br />
of basal implant systems, some changes in the<br />
design took place: earlier designs (approx. 1988–1999)<br />
had sandblasted endosseous surfaces. During this<br />
period, crater-like bone loss was reported to occur in<br />
a considerable amount of cases. Later, the vertical<br />
implant portions were polished, and only the baseplates<br />
remained sandblasted (1999–2003). Since 2003,<br />
all basal implants have been supplied completely<br />
polished; no related complications have been reported<br />
since. Polished surfaces resist colonialization by<br />
bacteria and the accumulation of plaque. They are<br />
Fig. 3b View of the lateral right side of the mandible (implantation<br />
procedure). Site 44 – threaded neck; Sites 45, 46, 47 –<br />
polished neck.<br />
Fig. 4b OPG – Two years and eight months after insertion.<br />
the standard of care in the treatment of fractures<br />
and meet the requirements for uneventful, stable<br />
osseointegration [3,10].<br />
Our cases with implant/restoration systems in<br />
heavily resorbed areas of the distal mandible sometimes<br />
revealed considerable new bone formation<br />
below and above the load-transmitting disks of basal<br />
implants. Bone formation is clearly driven by function<br />
and may be initiated by the surgical intervention and<br />
the interruption of the pre-existing bone trajectories.<br />
In implants with polished surfaces in the border area<br />
between the oral environment and the bone, vertical<br />
bone development is possible because infection has<br />
little chance of gaining ground.<br />
Fig. 5<br />
Progressive<br />
bone loss<br />
around crestal<br />
implants.
Fig. 6a OPG – Totally edentulous maxilla and mandible<br />
reconstructed with crestal and BOI implants using immediate<br />
loading protocol.<br />
Fig. 7a OPG – BOI implants inserted at sites 14, 17, 24, 27, 37,<br />
34, 44, 47.<br />
To clarify the causes of bone loss and to explore the<br />
possibility to use thinner and polished implant necks<br />
and transition portions, further research is required.<br />
Our cases of implant/restoration systems in heavily<br />
resorbed areas of the distal mandible sometimes<br />
revealed considerable new bone formation below and<br />
above the load-transmitting disks of BOI implants.<br />
Case 2 – male, 59 years old<br />
Clearly visible vertical bone growth at implant sites 37<br />
and 47, while marginal bone loss occurred around all crestal<br />
implants, particularly in the mandible (Figs. 6a and b).<br />
Case 3 – female, 48 years old<br />
Clearly visible vertical bone growth at all mandibular<br />
implant sites as well as at sites 17 and 27 (Fig. 7a). Figure<br />
7b shows the 5-year postoperative view.<br />
We have not yet developed a research plan; we are<br />
introducing this patient data as indicative for particular<br />
discussion. The results of our clinical work are<br />
presented to help define future research needs.<br />
Conclusions<br />
From the data analyzed in four relevant studies on<br />
bone loss following implant insertion, it can be con-<br />
<strong>EDI</strong> 61<br />
Clinical Science<br />
Fig. 6b OPG – Two years and nine months after insertion.<br />
Fig.7b OPG – Four years and ten months after insertion.<br />
cluded that there is less bone loss around the<br />
implants with threaded necks. Reported differences<br />
were statistically significant as far as marginal bone<br />
loss was concerned when implants with polished<br />
necks were compared to implants with microthreads.<br />
Our cases were presented in order to support this<br />
conclusion, even though the small sample size precludes<br />
statistical analysis. Further research is needed<br />
to obtain proof of the amount of marginal bone loss<br />
in long-term function after implant insertion when<br />
implants with machined and microthreaded neck are<br />
followed up and compared. In addition, our cases of<br />
implant/restoration systems in heavily resorbed<br />
areas of the distal mandible sometimes revealed considerable<br />
new bone formation below and above the<br />
load-transmitting disks of BOI implants.<br />
A list of references can be found at www.teamwork-media.de<br />
Contact address<br />
Vitomir S. Konstantinovic, DDS, MD, MSci, PhD<br />
Professor of Maxillofacial Surgery and Implantology<br />
University of Belgrade, School of Dentistry,<br />
Clinic of Maxillofacial Surgery<br />
Dr Subotica 4 · 11000 Belgrade · Serbia<br />
Phone/Fax: +381 11 2685342<br />
v.konstantinovic@stomf.bg.ac.rs
Institute<br />
The Eduardo Anitua Institute (Vitoria, Spain)<br />
is one of the outstanding international<br />
training centres in the fields of implantology<br />
and oral rehabilitation. Supported by most<br />
modern medical and audiovisual technologies<br />
you experience advanced training on highest<br />
level, scientific and practice oriented.<br />
The centre includes an auditorium for 74<br />
persons, several seminar rooms and an<br />
additional training room for practical<br />
exercises.<br />
Advanced Training<br />
The desired live-surgery courses of Dr. Eduardo<br />
Anitua are offered several times per year. The<br />
capabilities of the attendants get improved in the<br />
most important areas of implantology by livesurgeries,<br />
practice oriented lectures and handson<br />
exercises.<br />
For further information<br />
please contact:<br />
BTI Biotechnology Institute<br />
San Antonio 15 - 5º<br />
01005 Vitoria (ALAVA) • SPAIN<br />
BTI<br />
Training Center<br />
www.eduardoanitua.com<br />
Tel.: (34) 945 149 202<br />
Fax: (34) 945 154 909<br />
export@bti-implant.es
BTI of North America<br />
1730 Walton Road<br />
Suite 110<br />
Blue Bell, PA 19422-1802<br />
USA<br />
Tel: (1) 215 646-4067<br />
Fax: (1) 215 646-4066<br />
info@bti-implant.us<br />
BTI EXPANDER IMPLANTS<br />
Alternative and perfect complement to the standard crest expansions<br />
Motorized expanders Expander implants ____________________________________________________________<br />
Expander implants are used as a bridge between BTI expander kit and the definitive dental<br />
implants, achieving a most efficient crest expansion.<br />
Motorized expander kit Expander implant range<br />
BTI Deutschland GmbH.<br />
Mannheimer Str. 17<br />
75179 Pforzheim<br />
GERMANY<br />
Tel: (49) 7231 428060<br />
Fax: (49) 7231 4280615<br />
info@bti-implant.de<br />
BTI Biotechnology UK Ltd.<br />
870 The Crescent<br />
Colchester Business Park<br />
Colchester • Essex<br />
CO49YQ UNITED KINGDOM<br />
Tel:(+44)01206580160<br />
Fax:(+44)01206580161<br />
info@bti-implant.co.uk<br />
BTI Implant Italia Srl.<br />
Piazzale Piola n.1<br />
20131 Milano<br />
ITALY<br />
Tel.: (39) 02 70605067<br />
Fax: (39) 02 70639876<br />
bti.italia@bti-implant.it<br />
They are an excellent option<br />
when restoring the alveolar<br />
process in very reabsorbed<br />
crests between 2.5 and 3.5<br />
mm. remaining bone width.<br />
BTI de México<br />
E. N. Mexicano 351 piso 3A<br />
Col. Granada. Delegación Miguel<br />
Hidalgo<br />
Mex DF CP 11520 • MEXICO<br />
Tel: (52) 55 52502964<br />
Fax: (52) 55 55319327<br />
bti.mexico@bti-implant.com<br />
San Antonio 15 - 5º<br />
01005 Vitoria (ALAVA)<br />
SPAIN<br />
Tel.: (34) 945 140 024<br />
Fax: (34) 945 135 203<br />
export@bti-implant.es<br />
www.bti-implant.com / www.prgf.org<br />
BTI Portugal<br />
R. Pedro Homem de Melo<br />
55 S/6.03<br />
4150-000 Porto • PORTUGAL<br />
Tel: (351) 22 618 97 91<br />
Fax: (351) 22 610 59 21<br />
bti.portugal@bticomercial.com
64 <strong>EDI</strong><br />
Clinical Science<br />
Implant abutments created by CAD/CAM<br />
Roads to implant abutments<br />
Josef Schweiger, MDT, Professor Daniel Edelhoff, PD Dr Florian Beuer and<br />
Dr Michael Stimmelmayr, Munich/Germany<br />
The development of CAD/CAM technology in recent years has opened up many new options for customizing implant<br />
abutments [9,10]. Most attention has been devoted to titanium and zirconia [11]. Not getting lost in a host of new fabrication<br />
techniques and design principles has become increasingly difficult for today’s users. The purpose of this review is to supply<br />
an overview of current approaches to fabricating custom implant abutments.<br />
Implant abutments constitute the critical interface<br />
between osseointegrated implants and their prosthetic<br />
superstructure. They embody the sensitive<br />
transition from the implant through the peri-implant<br />
soft tissue to the oral cavity and the prosthetic<br />
restoration. Implant abutments therefore need to<br />
meet specific requirements, including high stability,<br />
long-term strength and resistance to chemical<br />
action. They need to offer excellent biocompatibility<br />
and the ability to customize their shape and angulation.<br />
The exact nature of these requirements will<br />
depend on the implant site along the arch.<br />
Customized emergence profiles and shades and<br />
translucencies resembling those of natural teeth are<br />
considered essential to the reconstruction of satisfactory<br />
(notably anterior) aesthetics in the presence of A1<br />
or A2 periodontal morphotypes [1,2]. In addition, toothcoloured<br />
materials in the aesthetic zone will also be<br />
beneficial in the event of adverse structural conditions<br />
giving rise to abutment exposure. Initial attempts to<br />
solve these exposure-related problems were sporadically<br />
made by layering sintered ceramics over titanium<br />
abutments, laying the foundation for subsequent<br />
delivery of all-ceramic restorations (Figs. 1 and 2).<br />
1 2<br />
Fig. 1 Build-up of a titanium abutment (Steri-Oss) with sintered<br />
porcelain to accommodate a glass-ceramic crown with<br />
a lithium-disilicate framework.<br />
In 1993, Prestipino and Ingber presented a densely<br />
sintered abutment made of alumina, to be used as an<br />
all-ceramic alternative to metal-based abutments<br />
in the anterior segment [3]. After taking a direct<br />
impression at implant level, cylinders of highly pure<br />
and densely sintered alumina ceramic cylinders were<br />
prepared, usually for restoration with all-ceramic<br />
crowns. Some of the laboratory procedures were very<br />
time-consuming. At the same time, the minimum<br />
thickness of the material could not be adequately<br />
controlled. Veneering ceramics fused to the surface<br />
were used for additive customization to establish an<br />
adequate emergence profile (Fig. 3). An unprecedented<br />
level of aesthetics and light transmission was<br />
achieved by combining these abutments with glassceramic<br />
crowns (Figs. 4 to 7).<br />
Soon after the introduction of alumina abutments,<br />
the first experimental abutments made from<br />
partially stabilized zirconia became available. Like<br />
their alumina predecessors, these zirconia abutments<br />
would involve a time-consuming manual<br />
process of customization from the prefabricated<br />
cylinders in the dental laboratory (Figs. 8 and 9). The<br />
new zirconia abutments featured both a radiopacity<br />
Fig. 2 Situation after delivering the glass-ceramic crown to<br />
the veneered abutment (technician: Volker Weber, Aachen,<br />
Germany).
Fig. 3<br />
All-ceramic<br />
implant abutment<br />
on a master<br />
cast with<br />
artificial gingiva.<br />
The abutment<br />
was made from<br />
highly pure and<br />
densely sintered<br />
alumina ceramic<br />
(Ceradapt, Nobel<br />
Biocare). Its<br />
emergence profile<br />
was modified<br />
(technician:<br />
Volker Weber,<br />
Aachen, Germany)<br />
with sintered<br />
ceramics<br />
(Vitaduralpha,<br />
Vita Zahnfabrik).<br />
Fig. 7<br />
All-ceramic<br />
implant restoration.<br />
Other than<br />
a mild shadow<br />
from the gold<br />
screw, light<br />
transmission is<br />
similar to a<br />
natural tooth.<br />
3 4<br />
5 6<br />
Fig. 5 Alumina abutment (Ceradapt) after connection with a<br />
torque driver.<br />
7<br />
8 9<br />
Fig. 8 Cylindrical blank of HIP zirconia (Wohlwend Innovative,<br />
Zurich, Switzerland) for abutments to be used with Brånemark<br />
implants.<br />
similar to metals and a reduced hardness compared<br />
to the alumina variant [4]. An investigation performed<br />
in vitro demonstrated that these zirconia<br />
Fig. 4 Brånemark implant (Nobel Biocare) placed at site 11.<br />
<strong>EDI</strong> 65<br />
Clinical Science<br />
Fig. 6 Situation following adhesive delivery of a leucitereinforced<br />
crown (IPS Empress) in 1998 (technician: Andreas<br />
Rübben, Aachen, Germany).<br />
Fig. 9 Zirconia abutments after time-consuming customization<br />
for all-ceramic crowns.<br />
abutments had a fracture strength approximately<br />
2.5 times higher than that of their alumina counterparts<br />
[4].
66 <strong>EDI</strong><br />
Clinical Science<br />
10 12<br />
11<br />
Fig. 11 Experimental use of all-ceramic abutments (zirconia and alumina in the<br />
third and fourth quadrants respectively) in the posterior mandible (year: 1998).<br />
The highest fracture strength of metal-ceramic<br />
and all-ceramic crowns is, however, still observed on<br />
titanium abutments [5,6]. Long-term clinical investigations<br />
confirmed that the stability of zirconia abutments<br />
was higher than that of alumina abutments<br />
[7]. Similar findings were obtained among our own<br />
patients (Figs. 10 to 13). The literature reveals a beneficial<br />
effect on peri-implant soft-tissue conditions, as<br />
less bacterial adhesion was demonstrated on zirconia<br />
healing caps than on titanium ones [8].<br />
The development of CAD/CAM technology in<br />
recent years has opened up many new options for<br />
customizing implant abutments [9,10]. Most attention<br />
has been devoted to titanium and zirconia [11].<br />
Implant abutments have also benefited from the<br />
numerous improvements brought about by advanced<br />
manufacturing techniques. This progress has led to<br />
standardized fabrication routines, industrial-grade<br />
prefabrication of high-quality restorative materials<br />
and automated control of minimum layer thickness.<br />
Compared to manual fabrication, the processing routines<br />
have become more conservative and faster. Not<br />
getting lost in a host of new fabrication techniques<br />
and design principles has become increasingly difficult<br />
for today’s users. The purpose of this review is to<br />
supply an overview of current approaches to fabricating<br />
custom implant abutments.<br />
13<br />
Fig. 13 The implant was extensively reduced with abrasive<br />
instruments for correct axial inclination. Given the means<br />
available in laboratories at the time, minimum thickness of<br />
the material could not be adequately controlled.<br />
Fig. 10<br />
Experimental<br />
use of all-ceramic<br />
abutments<br />
(alumina and<br />
zirconia in the<br />
first and second<br />
quadrants<br />
respectively)<br />
in the posterior<br />
maxilla (year:<br />
1998).<br />
Fig. 12<br />
Fracture of an<br />
experimental<br />
alumina abutment<br />
(Ceradapt,<br />
Nobel Biocare)<br />
at site 16 following<br />
four years of<br />
clinical service.
Tab. 1 General classification of implant abutments<br />
Prefabricated abutments<br />
Customizable<br />
Non-customizable<br />
Cast-to/press-to abutments<br />
Cast-to HSL sleeves<br />
Press-to CoCr abutments (POC)<br />
CAD/CAM implant abutments<br />
Titanium<br />
All-ceramics (with or without bonding base)<br />
CoCr alloy (Wirobond Mi+, for Bego Semados)<br />
Classification of abutments<br />
A general classification of implant abutments can<br />
be made by fabrication techniques, distinguishing be -<br />
tween prefabricated abutments, castable/pressable<br />
abutments and CAD/CAM implant abutments (Tab. 1).<br />
Implant manufacturers offer prefabricated abutments<br />
in various sizes, shapes and angulations. Also, they<br />
are available as customizable and non-customizable<br />
versions. However, since the limits of customization<br />
are quickly reached with prefabricated abutments,<br />
users have long desired to modify the shape of abutments<br />
as freely as possible. An intermediary solution<br />
was provided in the form of HSL sleeves. These were<br />
designed so laboratory technicians can wax up an<br />
ideal geometry, which is then implemented in a precious<br />
metal using the lost-wax technique (Fig. 14).<br />
Alternatively, pressable ceramics can be used for custom<br />
shaping, thus yielding press-on ceramic (POC)<br />
abutments. Oversized customizable abutment<br />
blanks (e.g. Telescope Abutment, Camlog Biotechnologies,<br />
Basel, Switzerland) would be another<br />
option (Fig. 15). However, the effort that goes into processing<br />
these blanks is considerable. In addition, this<br />
type of customization involves a risk of inappropriate<br />
handling or damage to the material (whether titanium<br />
or zirconia) by overheating. Customized zirconia<br />
blanks should therefore be processed only under irrigation<br />
with copious amounts of water. To allow customization<br />
of titanium abutments under irrigation,<br />
one manufacturer (Komet, Brasseler, Lemgo, Germany)<br />
has developed a specialized set of cutters (original<br />
design by J. H. Bellmann) for use in irrigated laboratory<br />
14<br />
15<br />
16<br />
<strong>EDI</strong> 67<br />
Clinical Science<br />
Fig. 14 Castable sleeves featuring an HSL base are currently<br />
the most popular approach to fabricating custom abutments.<br />
Figs. 15 and 16 Prefabricated customizable abutment (Telescope<br />
Abutment, Camlog Biotechnologies).<br />
turbines (Fig. 16). This system greatly improves the<br />
performance of material reduction while minimizing<br />
heat development. CAD/CAM abutments are predominantly<br />
fabricated from titanium and zirconia [12].<br />
While titanium abutments are processed as mono -<br />
blocks, all-ceramic abutments may be used either<br />
with or without a bonding base.
68 <strong>EDI</strong><br />
Clinical Science<br />
17 18<br />
19<br />
1. Abutments implemented by CAD/CAM from<br />
titanium monoblocks<br />
Figures 17 and 18 illustrate this concept. Abutments<br />
implemented by CAD/CAM from titanium<br />
are offered by various manufacturers. For the time<br />
being, the computer-assisted manufacturing of these<br />
abutments can only be performed in dedicated<br />
milling centres (e.g. Straumann CAD/CAM, Leipzig,<br />
Germany; Nobel Procera, Göteborg, Sweden; Compartis<br />
ISUS, Hanau, Germany; Astra Atlantis, Möln -<br />
dal, Sweden). Manufacturers either use industrially<br />
prefabricated blanks of a semi-finished nature (i.e.<br />
with the implant-abutment interface already prefabricated<br />
to industrial standards) or finished abutments<br />
milled from a titanium block (i.e. with both<br />
the external shape and the interface already completed)<br />
for CAD/CAM implementation. Ready-made<br />
CAD/CAM abutments offer the advantage of not<br />
requiring any subsequent processing other than surface<br />
polishing with silicone rubber cups if required.<br />
This eliminates the need for expensive corrections<br />
and avoids the risk of damaging the material in the<br />
process.<br />
2. Abutments implemented by CAD/CAM from<br />
zirconia<br />
Zirconia CAD/CAM abutments may be fabricated with<br />
or without a titanium bonding base. Both variants<br />
have their specific advantages and disadvantages.<br />
3a. Ceramic abutments without a bonding base<br />
Like titanium abutments, those ceramic abutments<br />
that do not use a bonding base are also implemented<br />
from monoblocks by CAD/CAM (Fig. 19) [13]. They<br />
are also supplied as semi-finished blanks with the<br />
abutment-implant interface already prefabricated in<br />
an industrial process. The external abutment geometry<br />
is custom-milled from the blank, utilizing the<br />
design data or the wax-up that the laboratory has<br />
generated for this purpose. CAD/CAM ceramic abutments<br />
without a bonding base have both advantages<br />
and disadvantages. A brief summary of these follows.<br />
Advantages:<br />
• With no subsequent adjustments performed to<br />
the abutments, there is no risk of inflicting damage<br />
to the material.<br />
• White (i.e. tooth-coloured) abutments will offer<br />
aesthetic advantages for anterior implant restorations.<br />
• Perfect biocompatibility in the sensitive transition<br />
zone from implant to abutment.<br />
Disadvantages:<br />
• No conical anchorage of the abutment screws, as<br />
these screws have direct contact with the ceramic<br />
material, thus being usually anchored in a perpendicular<br />
channel with parallel walls to avoid tensile<br />
stresses inside the ceramic abutment. These con-<br />
Figs. 17 and 18<br />
Titanium<br />
monoblock<br />
abutments.<br />
Fig. 19<br />
CAD/CAM<br />
ceramic abutment<br />
(without a<br />
bonding base).
Figs. 20 and 21<br />
CAD/CAM<br />
ceramic abutment<br />
(with a<br />
bonding base).<br />
Fig. 22<br />
Screw designs for<br />
titanium monoblock<br />
abutments,<br />
all-ceramic abutments<br />
without a<br />
bonding base and allceramic<br />
abutments<br />
with a bonding base<br />
(left to right).<br />
Different screw<br />
designs are usually<br />
employed for these<br />
abutment types.<br />
20 21<br />
22<br />
nections therefore carry a higher risk of screw<br />
loosening than the ones used in titanium abutments.<br />
• Ceramic abutments will generally involve a higher<br />
risk of facture than titanium abutments.<br />
3b. Ceramic abutments with a bonding base<br />
All-ceramic CAD/CAM implant abutments with a<br />
bonding base are fabricated from presintered zirconia<br />
either in a dental laboratory (e.g. inLab MCXL,<br />
Sirona, Bensheim, Germany; Everest, KaVo, Biberach,<br />
Germany) [12,14] or in a milling centre (e.g. Bego Medical,<br />
Bremen, Germany; 3M Espe Lava, Seefeld, Germany)<br />
(Figs. 20 and 21). One approach is to use partially<br />
prefabricated “mesioblocks” of zirconia, designed<br />
as semi-finished blanks with the connection from<br />
abutment to bonding base already incorporated.<br />
Once the external geometry has been ground to<br />
shape, the abutments are densely sintered and connected<br />
to the bonding base. The bonding bases as<br />
such are industrially manufactured from titanium,<br />
hence offering the same fit as prefabricated titanium<br />
abutments. Again, a number of advantages and disadvantages<br />
should be considered.<br />
Advantages:<br />
• Conical anchorage of the abutment screw inside<br />
the titanium bonding base, which reduces the risk<br />
of screw loosening (Fig. 22).<br />
<strong>EDI</strong> 69<br />
Clinical Science<br />
• Tightening the connection screws will not introduce<br />
any tensile stresses into the ceramic abutment.<br />
• Can be fabricated outside of milling centres<br />
(i.e. in laboratories and dental offices).<br />
• Low cost.<br />
Disadvantages:<br />
• Additional effort of bonding the ceramic abutment<br />
to the titanium base.<br />
• Risk of debonding if inappropriately executed.<br />
• Reduced quality management by lack of industrial<br />
manufacturing.<br />
• Increased affinity to plaque formation (due to the<br />
bonding gap filled with composite) in the sensitive<br />
transition zone from implant to abutment.<br />
4. Abutments implemented by CAD/CAM from CoCr<br />
alloy and precious metal on a titanium bonding base<br />
Bego additionally offers an CoCr alloy (Wirobond C+,<br />
Bego) as metal material for CAD/CAM bonding abutments.<br />
All CAD/Cast materials are also offered by this<br />
manufacturer. In the CAD/Cast process, resin restorations<br />
are fabricated in the milling centre based on<br />
the CAD designs generated by the laboratory technician.<br />
These resin restorations are then cast using the<br />
lost-wax technique in an industrial process of vacuum<br />
pressure casting. Eight different precious alloys<br />
and one non-precious alloy (Wiron 99, Bego) are currently<br />
available for this purpose.
70 <strong>EDI</strong><br />
Clinical Science<br />
23 24<br />
25 26 27<br />
Three strategies of implementing abutments<br />
by CAD/CAM<br />
Three different fabrication methods for CAD/CAM<br />
abutments can be distinguished at the present time:<br />
• Complete outsourcing (e.g. AstraTech Atlantis).<br />
• CAD in the laboratory, CAM in the milling centre<br />
(e.g. Straumann Wax Abutment, Straumann Cares,<br />
Bego Medical).<br />
• CAD in the laboratory, CAM in the laboratory (e.g. KaVo<br />
Neolink, Sirona zirconia bonding abutment) [14].<br />
1. Complete outsourcing<br />
Examples of complete outsourcing of implant abutments<br />
include Astra Atlantis (Astra Tech, Mölndal,<br />
Sweden) and abutments supplied by the Compartis<br />
ISUS milling centre (DeguDent, Hanau, Germany).<br />
This implementation strategy will be outlined using<br />
the Atlantis system (Astra Tech) as an example.<br />
The first step after fabricating the implant model<br />
(Figs. 23 and 24) is to create a wax-up. The adaptation<br />
of prefabricated teeth has proved to be highly efficient<br />
for this purpose. To achieve optimal positioning of the<br />
teeth over the implant analogues on the cast, they can<br />
be waxed up to length-reduced impression posts for<br />
reproducible placement and removal (Figs. 25 to 27).<br />
The abutments are defined and ordered through a Webbased<br />
form (Figs. 28 and 29). A number of design features<br />
for the CAD/CAM abutment to be customized<br />
can be entered. These include parameters such as<br />
transmucosal profile (Fig. 30), preparation depth as<br />
measured from the gingival margin or implant shoulder,<br />
shape of the abutment preparation (chamfer or<br />
shoulder), any retention surfaces in the case of titanium<br />
abutments and any parallelism between abut-<br />
ments if desired (Fig. 31). With regard to mucosal shaping,<br />
for example, these four design options are available:<br />
• Mucosa will not be shaped.<br />
• Mucosa will be supported.<br />
• Mucosa will be shaped.<br />
• Maximum anatomical width.<br />
A parcel service takes care of collection and delivery.<br />
Delivery of titanium abutments is guaranteed within<br />
five days; delivery of zirconia abutments within seven<br />
days. Orders of more than three abutments will delay<br />
the shipment by one day per additional unit.<br />
The customized abutments are fabricated based<br />
on the existing wax-up in the milling centre. Astra<br />
Tech’s patented software solution Atlantis VAD (Virtual<br />
Abutment Design) permits matching the abutment<br />
design to the definitive external geometry of<br />
the restoration. The resultant design will greatly facilitate<br />
the subsequent task of creating the framework<br />
and veneer back in the laboratory. Framework errors<br />
are virtually ruled out by this procedure. Once the<br />
abutments have been designed, screenshots are sent<br />
by e-mail for verification (Fig. 32). In early 2010, a<br />
service was introduced that allows technicians to<br />
verify and check the CAD abutments with a 3D viewer<br />
(Fig. 33). The go-ahead for the actual fabrication<br />
process is also given by e-mail.<br />
A collaborative effort by 3M Espe and Astra Tech<br />
has resulted in a new approach based on the Lava<br />
Scan ST system. After scanning laboratory-fabricated<br />
implant models with this system, the data captured<br />
are transmitted directly to the milling centre. This new<br />
procedure enables laboratories that use Lava Scan ST<br />
to place orders without having to ship a model to<br />
Astra Tech (Figs. 34 to 36).<br />
Figs. 23 and 24<br />
Implant master<br />
cast with artificial<br />
gingiva and<br />
two implants<br />
(Astra Osseo<br />
Speed) at sites 35<br />
and 36.<br />
Fig. 25<br />
Length-reduced<br />
impression posts<br />
can be used to facilitate<br />
the set-up.<br />
Figs. 26 and 27<br />
The set-up can be<br />
performed with<br />
prefabricated teeth,<br />
which are cut back<br />
to fit the reduced<br />
impression posts.
Fig. 28<br />
The order is<br />
placed via the<br />
Atlantis website.<br />
Fig. 29<br />
Input form collecting<br />
the case<br />
data and design<br />
parameters for<br />
the various<br />
implants.<br />
Fig. 30<br />
Various design<br />
parameters can be<br />
entered for the<br />
custom CAD/CAM<br />
abutments (e.g.<br />
mucosal shaping).<br />
Fig. 31<br />
Several different<br />
abutments can be<br />
designed in<br />
parallel.<br />
Fig. 32<br />
Screenshots are<br />
sent by e-mail<br />
for verification<br />
of the designs.<br />
Fig. 33<br />
A 3D viewer is<br />
offered to verify<br />
the abutment<br />
design.<br />
Figs. 34 to 36<br />
Lava Scan ST<br />
(3M Espe) offers<br />
scanning of<br />
implant models<br />
created in the<br />
laboratory and<br />
direct transfer of<br />
the resultant<br />
data to Astra<br />
Tech (Mölndal,<br />
Sweden).<br />
28 29<br />
30 31<br />
32 33<br />
34 35<br />
36<br />
<strong>EDI</strong> 71<br />
Clinical Science
72 <strong>EDI</strong><br />
Clinical Science<br />
37<br />
38 39<br />
Another collaborative effort by Astra Tech and Dental<br />
Wings (Montreal, Ontario, Canada) has been<br />
under way since early 2010. A similar principle as with<br />
the 3M Espe scanner is used as data from the Dental<br />
Wings 3D scanner can be directly transmitted by<br />
electronic means to Astra Tech’s design and milling<br />
centre for fabrication of Atlantis abutments.<br />
Atlantis abutments are available with interfaces<br />
for different implant systems (Astra Tech, Nobel Biocare,<br />
Straumann, 3i, Lifecore, Zimmer Dental, Biohorizons,<br />
Innova and Sterngold). They are offered as zirconia,<br />
titanium or GoldHue (titanium nitride-coated<br />
titanium) abutments (Figs. 37 to 39).<br />
2. CAD in the laboratory, CAM in the milling centre<br />
Another way of obtaining CAD/CAM abutments is<br />
to perform the CAD (i.e. the design) part in the laboratory<br />
and then to transfer the data to a milling<br />
centre electronically for fabrication of the customized<br />
abutments with industrial milling systems<br />
based on the data record from the laboratory. Two<br />
different techniques are available to generate the<br />
CAD record:<br />
• Creating a wax abutment.<br />
• Designing the abutment by CAD only.<br />
2a. Wax abutment<br />
This option is currently available, for instance, with<br />
the Straumann CAD/CAM system. The Straumann<br />
Wax Abutment will serve as an example to outline<br />
this implementation strategy. Wax-up sleeves are<br />
used as basis for the wax abutments (Fig. 40). These<br />
sleeves facilitate the task of waxing up the abutment<br />
geometry; in addition, they also serve as connection<br />
element to the scan mount in the Straumann ES-1<br />
Scanner (Figs. 41 to 44) [3,9]. Upon completion of<br />
scanning, the system will generate a CAD record of<br />
the wax-up. Subsequent modifications to the design<br />
such as smoothing of surface irregularities can be<br />
performed (Figs. 45 and 46). Finally, the data record is<br />
transferred to the Straumann milling centre (Leipzig,<br />
Germany) for fabrication of a monoblock (i.e. without<br />
a bonding base) abutment. Advanced HSC (highspeed<br />
cutting) systems are available at the milling<br />
centre for this purpose (Fig. 47). The implant-abutment<br />
interface being a prefabricated component,<br />
only the external abutment surface needs processing.<br />
Zirconia abutments are fabricated from a hot isostatically<br />
pressed (HIP) material that offers superior<br />
mechanical properties due to industrial-grade sintering<br />
(Figs. 48 to 50).<br />
Figs. 37 to 39<br />
Atlantis abutments<br />
are<br />
offered in zirconia,<br />
titanium or<br />
GoldHue (titaniumnitride-coated<br />
titanium).
40<br />
43 44<br />
45 47<br />
46<br />
Figs. 45 and 46 Wax abutment as CAD<br />
record.<br />
41 42<br />
Fig. 47 The abutments are fabricated in the milling centre.<br />
48 49 50<br />
Figs. 48 to 50 Zirconia monoblock abutment to be used with a Straumann Bone Level RC implant.<br />
<strong>EDI</strong> 73<br />
Clinical Science<br />
Figs. 40 to 44 Wax-up sleeves facilitate the task of waxing up the abutment geometry; in addition, they also serve as connection<br />
element to the scan mount in the Straumann ES-1 scanner.
74 <strong>EDI</strong><br />
Clinical Science<br />
51 52<br />
53 54<br />
2b. Straumann Cares and Straumann CAD/CAM<br />
abutments<br />
This version of implementing CAD/CAM abutments<br />
involves a CAD record generated in the dental laboratory,<br />
which is then electronically transferred to the<br />
Straumann (Cares or CAD/CAM) milling centre. Cares<br />
or Straumann CAD/CAM abutments are designed with<br />
Cerec 3D (Figs. 51 to 55) or Etkon Visual (version 5.0 or<br />
later) software, respectively. CAD-based manipulation<br />
tools for abutments include rotation, scaling and translation.<br />
It is also possible to add and subtract material.<br />
The software ensures that predefined values for minimum<br />
wall thickness are respected. Sirona Cares, for<br />
example, outlines the minimum thickness in blue while<br />
the implant screw channel is displayed in red.<br />
The same principle of electronically transferring<br />
the CAD record to the milling centre once the design<br />
part has been completed also applies to the Straumann<br />
(Cares or CAD/CAM) system. The process in -<br />
volved corresponds to the Wax Abutment process.<br />
55<br />
3. CAD in the laboratory, CAM in the laboratory<br />
The third possibility is to conduct the entire fabrication<br />
process for custom CAD/CAM abutments in the<br />
dental laboratory. Titanium bonding bases are used<br />
for this purpose. The abutments milled from zirconia<br />
are bonded to these bases [14]. The blanks are custom-milled<br />
in a pre-sintered condition and therefore<br />
need to be densely sintered after milling. The following<br />
two solutions are available to fabricate the zirconia<br />
abutments:<br />
3a. Conventional zirconia blanks<br />
Here the zirconia abutment is milled from a conventional<br />
zirconia blank, such that the milling system<br />
will also take care of creating the screw canal. Examples<br />
include:<br />
• Titanium bonding bases and scan bodies by Neoss<br />
(Neolink) for the Everest system (Figs. 56 to 59) [15].<br />
• Titanium bonding bases and scan bodies by Prowital<br />
(Figs. 60 to 65) [14,16].<br />
Figs. 51 and 52<br />
CAD of a Straumann<br />
Cares<br />
abutment with<br />
Cerec 3D software.<br />
Fig. 53<br />
Fabrication of<br />
Straumann<br />
Cares abutments<br />
is centralized.<br />
Figs. 54 and 55<br />
Straumann<br />
Cares abutment<br />
in the patient’s<br />
mouth.
Figs. 56 to 59<br />
Neoss Neolink<br />
titanium bonding<br />
base and<br />
scan body for<br />
CAD with the<br />
KaVo Everest<br />
system.<br />
Figs. 60 to 62<br />
Titanium bases<br />
and scan bodies<br />
by Prowital.<br />
Figs. 63 to 65<br />
The implant<br />
module in<br />
3Shape Dental<br />
Designer is used<br />
for CAD.<br />
56<br />
58 57<br />
60 61<br />
62<br />
63 64 65<br />
57<br />
<strong>EDI</strong> 75<br />
Clinical Science
76 <strong>EDI</strong><br />
Clinical Science<br />
66 67<br />
Fig. 66 Camlog bonding base.<br />
• Titanium bonding bases by Medentika (Hügels -<br />
heim, Germany) of the 800 series in combination<br />
with an add-on module (nt-IQ by NT-Trading,<br />
Neustadt, Germany) for 3Shape Dental Designer.<br />
Scan bodies and bonding bases are available for<br />
numerous different implant systems.<br />
• Titanium bonding bases by NT-Trading (Neustadt,<br />
Germany). Scan bodies and bonding bases are<br />
available for numerous different implant systems.<br />
• Titanium bonding bases by Camlog are complemented<br />
by Camlog scan bodies (introduced in<br />
June 2010) to be used in dental CAD systems.<br />
• Titanium bonding bases, scan bodies and blanks<br />
(inCoris ZI meso) by Sirona are available for seven<br />
additional implant systems.<br />
3b. Semi-finished zirconia blanks<br />
Here the zirconia abutment is fabricated from a<br />
semi-finished blank. For this purpose, manufacturers<br />
are offering partially prefabricated blanks with the<br />
connection geometry of the bonding base already<br />
incorporated. All that remains to be done is to customize<br />
(by grinding/milling) the external surface to<br />
the desired shape. The blanks are milled in a pre-sintered<br />
condition and therefore need to be densely sintered<br />
after milling. To name two examples of semifinished<br />
zirconia blanks:<br />
Fig. 67 Semi-finished blank (Sirona inCoris ZI meso) before …<br />
68<br />
Fig. 68 … and after customization on the milling machine<br />
(Sirona inLab MCXL).<br />
• Camlog bonding base with Sirona (inCoris meso)<br />
blank (Figs. 66 to 71).<br />
• Sirona bonding bases, scan bodies and (inCoris<br />
ZI meso) blocks are available for seven additional<br />
implant systems.<br />
Most of the manufacturers listed use an anti-rotational<br />
mechanism of the interface between the titanium<br />
base and zirconia abutment, which ensures<br />
that the abutment will not rotate out of position to<br />
avoid incorrect bonding to the titanium base (Fig. 72).<br />
Overly tight seating of the abutment on the bonding<br />
base may be caused by interferences in the area of<br />
the anti-rotational mechanism, which can be precisely<br />
removed with a diamond cutter.<br />
The CAD process for abutments offers customary<br />
manipulation tools such as scaling, rotation, translation<br />
and adding/reducing material. Sirona Cerec/<br />
Sirona inLab introduced a CAD process for abutments<br />
in software revision V3.0. KaVo Everest has offered<br />
CAD/CAM abutments ever since version 3.x of the<br />
Energy software package was released. 3Shape Dental<br />
Designer is already capable of accommodating<br />
eleven different implant systems with the help of an<br />
add-on module (nt-IQ by NT-Trading) as well as scan<br />
bodies and titanium bonding bases (NT-Trading and<br />
Medentika). This option is supported, for instance, by
69 70<br />
Fig. 69 Individual components of a bonding abutment: titanium<br />
bonding base, abutment screw and custom-milled zirconia<br />
abutment.<br />
71 72<br />
Fig. 71 Bonding abutment in situ.<br />
the Bego CAD/CAM and 3M Espe Lava systems [15].<br />
The Heraeus Cara system offers this option since<br />
the second half of 2010. The bonding abutment is<br />
designed in the laboratory (3Shape Dental Designer,<br />
Lava design 5.0). The data record is then transferred<br />
to the milling centre, where the bonding abutments<br />
are fabricated and shipped back to the customer.<br />
Bego Medical currently offers zirconia (BeCe CAD<br />
Zirkon+, available in five shades; BeCe CAD Zirkon XH<br />
offering higher strength, available in three shades),<br />
CoCr (Wirobond C+) and all CAD/Cast materials for<br />
bonding abutments. In addition, Bego also offers to<br />
fabricate copings that will match the bonding abutments.<br />
Discussion<br />
The success of implant-supported restorations<br />
depends on a number of separate factors. One of<br />
these factors (alongside others such as bone situation,<br />
implant position, implant length and oral<br />
hygiene) is the abutment to be used with the<br />
implant. In many situations, prefabricated abutments<br />
will run up against limitations that will bring<br />
about dissatisfying outcomes. This is where custom<br />
CAD/CAM abutments come into their own [9]. They<br />
can be optimized in terms of axial inclination and<br />
Fig. 70 Bonding abutment after bonding.<br />
<strong>EDI</strong> 77<br />
Clinical Science<br />
Fig. 72 Manufacturers commonly use an anti-rotational<br />
mechanism between the titanium base and the zirconia.<br />
shape for existing situations. The most important<br />
clinical benefit of these abutments is their ability to<br />
shape the emergence profile. Used with cementable<br />
restorations, they eliminate any need for the tricky<br />
job of removing excess cement because the restorative<br />
margin can only be placed at (or slightly above)<br />
the gingival level. The effort of going into manual procedures<br />
remains within economically acceptable limits,<br />
and the materials used can be processed in a conservative<br />
fashion, which is not always the case with<br />
manual surface treatment of customizable abutment<br />
blanks. It is therefore not surprising that more<br />
and more implant manufacturers and CAD/CAM suppliers<br />
should join forces to provide their customers<br />
with the option of obtaining CAD/CAM abutments.<br />
These very positive developments will offer advantages<br />
for dentists and technicians. Most importantly,<br />
they will also benefit the patients.<br />
A list of references can be found at www.teamwork-media.de<br />
Contact address<br />
Josef Schweiger, MDT<br />
Poliklinik für Zahnärztliche Prothetik<br />
Klinikum der Universität München<br />
Goethestraße 70 . 80336 München . Germany<br />
zahn.labor@med.uni-muenchen.de
78 <strong>EDI</strong><br />
Clinical Science<br />
A new approach to ridge restoration in cases of severe horizontal resorption<br />
Transitional implants<br />
Eduardo Anitua, DDS, MD, Vitoria-Gasteiz/Spain<br />
The use of dental implants in the restoration of edentulous areas is standard practice today, with predictable outcomes [1].<br />
Sometimes, the recipient bone will be wide, high, and dense enough for implants to be placed without any further preparation.<br />
In patients who have been edentulous for a long time, however, we frequently encounter severe horizontal and vertical resorp-<br />
tion or a combination of defects that makes additional measures unavoidable (Figs. 1 and 2).<br />
In the case of horizontal problems, achieving satisfactory<br />
revascularization and a good prognosis requires<br />
at least 1 mm of bone width around the implant<br />
vestibularly and lingually/palatally [2]. When the<br />
width of the alveolar ridge to be restored is smaller<br />
than the diameter of the implant we propose to<br />
insert, we can resort to specific techniques for<br />
expanding the ridges horizontally [1,3,4].<br />
The principal techniques used for this purpose, as<br />
described in the literature, include: block bone grafts,<br />
particulate bone grafts with an absorbable or nonabsorbable<br />
mesh, bone substitute materials, ridge<br />
distraction systems and ridge expansion systems<br />
using expanders, osteotomes, and the split-crest<br />
technique using ultrasound or conventional surgery<br />
[1,3,5-8].<br />
Evolution of the split-crest technique<br />
The split-crest bone expansion technique, along with<br />
distraction, are the only techniques used for achieving<br />
horizontal growth that do not rely exclusively on<br />
grafts. It was first described by Simion et al. [7] in 1992<br />
for crests in which, despite severe horizontal resorption,<br />
the two plates were preserved intact and presented<br />
with a width of at least 4-5 mm.<br />
The technique allows the vestibular plate to be<br />
separated from the lingual or palatal plate, moving<br />
the vestibular cortical plate of the upper or lower jaw<br />
so as to separate it from the medullary cavity and to<br />
create an intermediate gap, which is then mostly<br />
filled by the implants inserted. The space not taken<br />
up by implants can be filled with biomaterials, particulate<br />
bone grafts or PRGF [8,9]. The space created<br />
between the two cortical plates then fills with new<br />
bone, enlarging the alveolar ridge so that techniques<br />
associated with increased morbidity (that require<br />
larger donor areas from which bone can be collected<br />
for grafting) can be dispensed with [4] (Figs. 3 and 4).<br />
The split crest technique has made considerable<br />
progress since it was first used. One of the major<br />
advances has been the introduction of ultrasound<br />
surgery for performing the osteotomy, replacing the<br />
conventional method using discs and chisels [9-11].<br />
Apart from jarring the patient with painful blows, the<br />
chisel method lacks precision and is difficult to control,<br />
especially in severely resorbed ridges with high<br />
bone density. Rotating or oscillating discs are less<br />
stressful for the patient but present significant limitations<br />
as regards accessibility, with possible injury to<br />
the lips, tongue and surrounding soft tissues [4].<br />
The electronic scalpel uses an ultrasound frequency<br />
that makes for great precision and safety when performing<br />
the osteotomy – a frequency that allows it<br />
to cut hard tissues such as bone without damaging<br />
other soft tissues such as gums, blood vessels, nerves<br />
or sinus membranes [4,9].<br />
In addition, the wide variety of specially designed<br />
tips means that the technique can be properly adapted<br />
to clinical situations involving different amounts<br />
of residual cortical bone. Another important point to<br />
emphasize is that the biological viability of ultrasound-treated<br />
bone is comparable to that of bone<br />
treated by other surgical techniques, and patients<br />
report less postoperative discomfort [11-13].<br />
More recently, the introduction of motorized<br />
expanders to replace the conventional manual<br />
osteotomy for enlarging the implant bed has helped<br />
shorten surgery times and reduce the complexity of<br />
the technique, making it more accessible to a larger<br />
number of professionals (Figs. 5 and 6).<br />
Gradual improvements to the conventional splitting<br />
technique now enable us to treat ridge crests<br />
with a width of 3.5 mm and upward, preserving both
1 2<br />
f<br />
e<br />
d<br />
cortical plates with excellent results. The challenge<br />
for us now is to be able to treat ridge crests 2.5 and<br />
3 mm in width.<br />
We begin by treating these cases with a technique<br />
called the “two-stage split ridge”. It is a variation on<br />
the conventional split-ridge technique, where, after<br />
osseointegration, the initial implant is replaced by<br />
another implant with a larger diameter.<br />
The implant is removed completely atraumatically<br />
using the new BTI extractor kit with a counter-torque<br />
device, which ensures that the peri-implant bed is<br />
kept intact after the explantation. Another drilling<br />
c<br />
b<br />
a<br />
<strong>EDI</strong> 79<br />
Clinical Science<br />
Figs. 1 and 2 Patterns of horizontal and vertical resorption in the mandible and maxilla as a consequence of tooth loss.<br />
Cases c and d in the mandible and b, c, and d in the maxilla look like obvious candidates for the transitional implant technique<br />
presented here.<br />
3 4<br />
a b c d e<br />
Figs. 3 and 4 Division of the ridge crest separating the vestibular and palatal cortical plates. Placement of the implants, showing<br />
the gap between the two plates that will be filled with new bone after a period of new bone formation.<br />
5 6<br />
Figs. 5 and 6 Using ultrasound to perform the split. Dilating the newly-formed socket by means of motorized expanders.<br />
procedure is then performed, and a further expansion<br />
step allows to place a larger-diameter implant,<br />
which in turn has the effect of expanding the ridge<br />
crest still further, while at the same time compacting<br />
the bone. In this way, more horizontal expansion is<br />
achieved than had been possible prior to the introduction<br />
of this new step, as seen in the clinical case<br />
treated in this way (Figs. 7 to 12).<br />
This technique has given excellent results, achieving<br />
an average enlargement of between 5 and 9 mm,<br />
depending on the measurement point (apical/coronal).<br />
There was just one challenge to overcome: when
80 <strong>EDI</strong><br />
Clinical Science<br />
Situation before Provisional implant Final implant<br />
7 8 9<br />
2,6<br />
2,6<br />
10 11 12<br />
Figs. 7 to 12 Images showing, from left to right, the progress of the case. The images on the left show a ridge crest marked by<br />
severe resorption and an average width of approximately 2.5 mm. The images in the centre show how much space is gained<br />
with the first split, doubling the width of the ridge crest. The images on the right show the final result after insertion of the<br />
second implant. We can see how the initial width has been tripled in size.<br />
using Tiny implants, which have a collar that opens<br />
out to 3.5 mm, we generally saw a vertical loss of<br />
1-2 mm of crestal bone in the area of the implant collar.<br />
We considered the possibility of using implants<br />
similar in design to the Tiny, but without the head,<br />
which had generated the bone loss by compression.<br />
These implants would allow us to proceed with a<br />
two-stage expansion with no loss in bone height.<br />
Moreover, these implants should have a surface<br />
treatment that would allow us not only to avoid the<br />
loss of crestal bone around the implant collar, but<br />
also, by exploiting the bioactive characteristics of the<br />
surface – coated with PRGF – to achieve vertical<br />
growth at this critical level.<br />
It was this thinking that led to the development of<br />
transitional implants that incorporate all these characteristics.<br />
They come in two lengths (8.5 and 11.5 mm)<br />
and two diameters (2.5 and 3 mm). They do not widen<br />
out at the collar and have a square implant mount<br />
connector, matching in size that of prosthetic screws,<br />
so that they can be inserted with the predesigned<br />
square tips using a contra-angle (Figs. 13 and 14).<br />
6,7<br />
5,6<br />
9,9<br />
10,9<br />
As a result, the advantage of this technique – which<br />
we could call the “accordion technique”, given that the<br />
expansion is performed in two stages – is that it<br />
allows us to make the two width adjustments that<br />
we described for the two-stage split technique, but<br />
without the risk of crestal bone loss seen previously.<br />
This reduction in diameter around the implant collar<br />
also gives us the option of carrying out a less pronounced<br />
separation of the plates at this level, which<br />
makes for better vascularization in the gap and, consequently,<br />
for a far more predictable result.<br />
At present, this technique lets us achieve horizontal<br />
growth of up to 300 per cent relative to the initial<br />
ridge crest, starting with crests as narrow as 2.5 mm,<br />
sometimes increasing to as much as 7 mm, thus<br />
often exceeding the results obtained with conventional<br />
horizontal enlargement techniques. The average<br />
width gain reported in different studies is equal<br />
to 3.6 mm, being somewhat better when using nonresorbable<br />
barrier membranes to cover the expansion<br />
site (4.2 mm) than when using resorbable membranes<br />
(2.9 mm) [3].
Fig. 13<br />
Family of<br />
transitional<br />
implants.<br />
Fig. 14<br />
Wetting a<br />
transitional<br />
implant with<br />
PRGF.<br />
13 14<br />
Lastly, we recommend filling the gap produced by<br />
the initial split and the first insertion of the transitional<br />
implant with activated PRGF and covering it<br />
with a fibrin membrane to ensure that the newly<br />
formed bone is of better quality and has an adequate<br />
blood supply. If we fill this gap with biomaterial,<br />
when we go back in to replace the transitional<br />
implant, we may find poorer-quality bone composed<br />
Clinical case 1<br />
1 2<br />
Fig. 1 Initial case photograph. Fig. 2 Initial X-ray.<br />
3 4<br />
Figs. 3 and 4 Image of the mandibular ridge after starting the split.<br />
<strong>EDI</strong> 81<br />
Clinical Science<br />
largely of the biomaterial originally placed there,<br />
which would seriously compromise osseointegration<br />
of the second implant and satisfactory vascularization<br />
of the bone bed.<br />
The clinical cases described below illustrate the use<br />
of transitional implants and the results achieved.<br />
A list of references can be found at www.teamwork-media.de
82 <strong>EDI</strong><br />
Clinical Science<br />
5 6<br />
Fig. 5 Inserting the transitional implant with a square tip.<br />
8 9<br />
10 11<br />
12 13<br />
7<br />
Fig. 6<br />
Transitional<br />
implants in<br />
place.<br />
Fig. 7<br />
X-ray after<br />
surgery.<br />
Figs. 8 and 9<br />
Re-entry after<br />
three months;<br />
note the gain in<br />
width (6 mm).<br />
Fig. 10<br />
CAT scans for diagnostic<br />
purposes. Photo showing<br />
a residual ridge<br />
2.5-3 mm in width and<br />
the placement of new,<br />
wider implants.<br />
Fig. 11<br />
Implant bed after<br />
removal of the<br />
transitional implants.<br />
Fig. 12<br />
The split must<br />
be covered with<br />
autologous<br />
fibrin.<br />
Fig. 13<br />
Final X-ray.
Figs. 1 to 3<br />
Initial case<br />
photographs.<br />
Fig. 4<br />
Initial X-ray.<br />
Figs. 5 and 6<br />
Measuring the<br />
width of the<br />
area to be<br />
treated.<br />
Note the width,<br />
which varies<br />
between<br />
2 and 3 mm.<br />
Fig. 7<br />
Situation after<br />
performing the<br />
split and before<br />
placing the<br />
implant.<br />
Fig. 8<br />
Implants<br />
placed.<br />
Clinical case 2<br />
1 3<br />
2<br />
4<br />
5 6<br />
7 8<br />
<strong>EDI</strong> 83<br />
Clinical Science
84 <strong>EDI</strong><br />
Clinical Science<br />
9 10<br />
11 12<br />
13 14<br />
15 16<br />
Contact address<br />
Eduardo Anitua Aldecoa, MD, DDS<br />
Calle Jose Maria Cagigal, 19 . 01007 Vitoria . Spain . eduardoanitua@eduardoanitua.com<br />
Figs. 9 and 10<br />
Photos showing<br />
a width of 6 mm<br />
around the<br />
transitional<br />
implants.<br />
Figs. 11 to 14<br />
CAT scans used<br />
for planning the<br />
handling of the<br />
case.<br />
Fig. 15<br />
Vestibular view<br />
of the implants.<br />
Note the<br />
implant heads<br />
remaining<br />
submerged<br />
in the ridge.<br />
Fig. 16<br />
X-ray after<br />
surgery.
Function, beauty and<br />
biology in perfect harmony<br />
Astra Tech BioManagement Complex <br />
The success of an implant system cannot be determined<br />
by one single feature alone. Just as with all<br />
natural systems, the delicate balance is maintained by<br />
the interaction of different but equally important<br />
features.<br />
Our implant system supports the natural balance by<br />
a unique combination of interdependent features—the<br />
Astra Tech BioManagement Complex . It is designed<br />
to ensure long-term clinical success by stimulating<br />
bone growth, providing bone preservation, soft tissue<br />
health and architecture. To put it simply: function,<br />
beauty and biology in perfect harmony.<br />
OsseoSpeed <br />
— more bone more rapidly<br />
MicroThread <br />
— biomechanical bone stimulation<br />
Conical Seal Design <br />
— a strong and stable fit<br />
Connective Contour <br />
— increased soft tissue contact zone and volume<br />
Astra Tech AB, Aminogatan 1, P.O. Box 14, SE-431 21 Mölndal, Sweden. Tel: +46 31 776 30 00. Fax: +46 31 776 30 10. www.astratechdental.com<br />
78786-USX-1004
86 <strong>EDI</strong><br />
Clinical Science<br />
Reconstruction of a case with maxillary atrophy by means of Short Implants<br />
and individual IACs (Integrated Abutment Crowns)<br />
A perfect fit<br />
Mauro Marincola 1 , Vincent Morgan 2 , Shadi Daher 3 , Sung-Kiang Chuang 4 and Angelo Perpetuini 5<br />
In the past, it was believed that osseointegrated dental implants needed to be at least 10 mm long for successful function.<br />
However, recent studies have shown that even shorter (< 10 mm) dental implants can perform well. Particularly, the plateau<br />
or fin design of dental implants with a bacterially sealed 1.5° locking-taper connection has provided for successful function<br />
of implants as short as 6.0 mm. Additionally, short unsplinted dental implants were associated with less crestal bone loss than<br />
longer splinted dental implants. This article reports on the placement and restoration of twelve ultra-short Bicon implants in<br />
conjunction with an internal sinus lift (by osteotome) and ridge-splitting procedures. The twelve 5.0 x 6.0 mm implants were<br />
aesthetically and successfully restored with single unsplinted Integrated Abutment Crowns in the severely compromised<br />
edentulous maxilla of a 58-year-old woman.<br />
In recent years, success criteria in oral implantology<br />
have included aspects other than the stability of the<br />
implant-prosthetic complex alone.<br />
Merely ensuring lasting bone integration of an<br />
implant is not all our patients need. Rather, the goal<br />
is a functional and aesthetic balance within the oral<br />
situation, consistent with the patient’s expectations.<br />
This can be achieved only by careful selection of the<br />
surgical and prosthetic techniques used.<br />
A physiological balance needs to be created be -<br />
tween the tissues at the recipient site and the implant-prosthetic<br />
complex – by eliminating the problems<br />
that have plagued oral implantology [1-4]:<br />
• Bacterial infiltration of the implant-abutment<br />
interface<br />
• Micro-movement at the level of the implant-abutment<br />
connection<br />
• Crestal bone resorption over time around the<br />
margin between the bone crest and the implant<br />
• Maladjustment of the gingival tissues around<br />
the emergence profile.<br />
The latest consensus statements on success criteria<br />
in oral implantology clearly show the need for long-<br />
1 Assistant Professor, University of Cartagena; private practice in Frankfurt and Rome.<br />
2 Clinical Director, Implant Dentistry Centre, Boston.<br />
3 Clinical Assistant Professor, Department of Maxillofacial Surgery, Boston University.<br />
4 Research Associate, Department of Biostatistics, Harvard School of Public Health, Boston.<br />
5 Master in Dental Technology, Rome.<br />
term preservation of both crestal bone structures and<br />
the soft tissues surrounding the restoration.<br />
Implants that maintain long-term stability but are<br />
affected by progressive bone loss with gingival irritation<br />
and recession can no longer be considered a<br />
success [5].<br />
The success of an implant should be judged by<br />
how successfully the crestal bone level around the<br />
implant is preserved over time. This is critical to<br />
ensure a supporting base and an adequate blood<br />
supply to the soft tissues surrounding the emergence<br />
point of the abutment/crown restoration. By<br />
keeping the crestal bone, the periosteum and the<br />
connective tissue and epithelial layer healthy and<br />
stable, undesirable situations can be avoided. Such<br />
situations would include crestal bone resorption and<br />
the development of peri-implantitis, which have been<br />
extensively documented in the literature [6].<br />
The clinical case presented here aims to illustrate<br />
how excellent results can be achieved that are<br />
highly attractive from an aesthetic point of view, at<br />
the same time ensuring the preservation of the crestal<br />
bone level surrounding the implant-prosthetic<br />
complex.
Fig. 5<br />
Enlargement of<br />
the osteotomies<br />
with latch<br />
reamers. Slow<br />
drilling (50 rpm<br />
without water<br />
irrigation) yields<br />
collectible<br />
autologous<br />
bone.<br />
Fig. 1 Severely atrophied maxilla with reduced vertical<br />
dimension in the posterior region.<br />
3 4<br />
Fig. 3 Elevating a full-thickness flap. Notice the thin bone<br />
layer at crest level.<br />
Fig. 4 Preparing for an osteotomy using a pilot drill, following<br />
the surgical guide and the paralleling pins.<br />
Clinical case<br />
The patient was a 58-year-old woman with a fully<br />
edentulous maxilla. Her medical history did not suggest<br />
any contraindications to implant therapy. The<br />
radiograph revealed severe atrophy of the maxillary<br />
bone, in particular in the posterior region (Fig. 1). The<br />
Fig. 2 Reduced frontal horizontal dimension.<br />
<strong>EDI</strong> 87<br />
Clinical Science<br />
clinical intraoral examination confirmed the presence<br />
of a thin alveolar crest in the anterior region (Fig. 2).<br />
The treatment plan originally provided for anterior<br />
ridge expansion using a synthetic bone graft plus<br />
resorbable membrane and a bilateral maxillary sinus<br />
lift. The patient did not consent to this last treatment,<br />
which made the use of Bicon Short Implants<br />
the alternative. Only 6 mm in length, these implants<br />
spare the patient invasive maxillary sinus procedures.<br />
The use of anaesthetic without vasoconstrictor<br />
enabled us to collect blood from the site intrasurgically<br />
(by means of a 5-ml sterile syringe), which was<br />
useful when it came to handling the graft material<br />
for crestal bone remodelling. After adrenaline anaesthesia,<br />
a full-thickness flap was elevated along the<br />
entire crestal bone perimeter (Fig. 3).<br />
Bicon’s surgical protocol requires the use of a 2 mm<br />
pilot drill that cuts only at the tip and perforates the<br />
cortical bone at 1000 rpm using external irrigation.<br />
The pilot drill defines the future depth of the single<br />
osteotomies. A surgical stent ensures the correct<br />
mesiodistal and buccolingual dimensions (Fig. 4).<br />
The surgeon then gradually widens the pilot<br />
osteotomies with mechanical reamers rotating at<br />
just 50 rpm without irrigating the surgical site.<br />
These reamers are special drills that do not cut at<br />
the tip, avoiding perforation or undesired collapse.<br />
Furthermore, they gather a considerable amount of<br />
autologous bone, subsequently used to cover im -<br />
plants seated below the crest (Fig. 5).
88 <strong>EDI</strong><br />
Clinical Science<br />
Fig. 6<br />
Implant insertion<br />
with seating<br />
tip. Notice<br />
the subcrestal<br />
position of the<br />
Ultra Short<br />
Implants.<br />
Fig. 8 Postoperative radiograph showing the homogenous<br />
placement of the upper implants without invasion of the vital<br />
structures. Note the immediately loaded mandibular implants.<br />
Once the osteotomies have been prepared, surgical-grade<br />
titanium alloy implants (Ti 6Al 4V) 5 mm in<br />
diameter and 6 mm in length are inserted by means<br />
of a seating tip. Each individual implant is tapped<br />
into place (Fig. 6). The neck of each implant must be<br />
placed 1-2 mm below the crest to ensure the cortical<br />
bone ridge is maintained once osseointegration and<br />
loading have occurred.<br />
In our case, implants 11, 12, 13, 21, 22 and 23 were<br />
inserted into the osteotomies following crestal bone<br />
expansion. Bone graft material was introduced, and<br />
the surgical site was covered with a resorbable collagen<br />
membrane. For the placement of implants 16<br />
and 26, an internal sinus lift technique was used; it is<br />
less invasive for the patient and easily carried out following<br />
the implant system protocol.<br />
The harvested bone collected during the reaming of<br />
the osteotomies was placed over the implant shoulders<br />
to assure complete crestal coverage of the im -<br />
plants (Fig. 7). Finally, the flap was sutured using<br />
resorbable sutures.<br />
The patient was recalled at seven and 21 days. The<br />
gingiva healed uneventfully. The removable denture<br />
was delivered and relined following the first postsurgical<br />
visit.<br />
Fig. 10 The twelve two-part impression posts show the perfect<br />
positioning of the implants. The locking-taper connection<br />
between the implant connection socket and the abutment<br />
post uses no screws, and no index is needed.<br />
After four months, the implants were uncovered.<br />
The radiographic control revealed the subcrestal position<br />
of the implants, preservation of the crestal bone<br />
during healing and the less invasive nature of the<br />
twelve ultra-short implants in relation to the atrophic<br />
crest (Fig. 8).<br />
The second surgical stage involved elevating a flap<br />
wide enough to uncover the healing abutments protecting<br />
the connector socket of the implant. The healing<br />
plugs were taken off and the impression posts<br />
inserted (Figs. 9 and 10).<br />
Fig. 7<br />
The implant<br />
shoulders are<br />
completely<br />
covered by the<br />
collected autologous<br />
bone.<br />
The black<br />
Macron healing<br />
plugs are protecting<br />
the<br />
implant well.<br />
Fig. 9<br />
Inserting<br />
impression posts<br />
four months<br />
after healing.<br />
Once a small<br />
flap is elevated<br />
and the healing<br />
abutments are<br />
removed, the<br />
posts are<br />
engaged into<br />
the connecting<br />
socket.
Fig. 11 The selected abutments are placed into the stone cast and moulded.<br />
Sandblasting is done after protecting the abutment posts with wax.<br />
Using the single-stage impression technique, the<br />
position of the implants was transferred from the<br />
oral cavity to the laboratory cast using elastomeric<br />
material. After applying the gingival material to the<br />
impression and pouring the impression in class IV<br />
stone, the lab employed the Integrated Abutment<br />
Crown (IAC) technique by selecting the abutments to<br />
be drilled. The crowns were modelled onto the prepared<br />
abutment surface, and chemical bonding of<br />
latest-generation polyceramic materials took place<br />
on the abutment itself (Integrated Abutment Crown).<br />
With no need for cementing, invasion of the biological<br />
space by non-biocompatible components was<br />
avoided and the gap between the aesthetic material<br />
and the prosthetic abutment was eliminated.<br />
The proper abutment selection for restoring Bicon<br />
implants depends on the respective tooth position.<br />
As the abutment post is inserted into the connector<br />
socket of the implant, a hemispherical base starting<br />
2 mm from the implant shoulder forms the emergence<br />
profile that maintains the soft-tissue margin.<br />
The diameters of the abutments are 6.5 mm for<br />
<strong>EDI</strong> 89<br />
Clinical Science<br />
molars, 3.5 mm for lower incisals, 4 mm for upper lateral<br />
incisors and 5 mm for canines, premolars and<br />
upper central incisors.<br />
The standard abutments are solid and have no<br />
fastening screws and can therefore be reduced as<br />
appropriate.<br />
In the case on hand, the gingival contour was<br />
marked with a felt-tip pen. The crown margin had to<br />
be placed 1-2 mm below the gingival contour, creating<br />
an individual crown/gingival anatomy for each<br />
IAC. The abutments required a rounded shape with<br />
no sharp edges, with a basal shoulder. It is vitally<br />
important that the hemispherical base is never modified<br />
and that the gingival tissues are kept in close<br />
contact with the titanium.<br />
During the sandblasting procedure and while<br />
primer agent and opaque paste were applied, the<br />
integrity of the abutment post was protected by<br />
placing it in a wax base (Fig. 11).<br />
Sandblasting was performed using aluminium<br />
oxide (50 μm), and the surface was cleaned with 95%<br />
ethanol in an ultrasonic bath for five minutes. The<br />
abutments were connected to metal implant analogues<br />
and treated with a layer of primer that wets<br />
the entire surface. Pre-opaque and opaque material<br />
of the chosen shade was then built up in layers and<br />
light-cured. The opaque dentine that replaces the<br />
metal frame had to support the following layers that<br />
determine stability and colour. The opaque dentine<br />
made up about 40 per cent of the final volume. Its<br />
layer thickness of 2 to 5 mm enabled the polyceramic<br />
material to spread across the surface of the abutments.<br />
The cervical materials, dentines, enamels and<br />
intensives used belonged to cervical group A and<br />
incisal group D on the Vita shade guide. These had to<br />
be built up such as to give the restoration a natural<br />
aesthetic look. The IAC crowns were finished using<br />
a silicon polishing wheel that removed any excess<br />
material between the abutment and polymer material,<br />
ensuring a perfect marginal seal. The advantage<br />
of this technique is that it avoids errors arising from<br />
fusion and cementation (Fig. 12).<br />
Fig. 12 Polyceramic material is chemically bonded onto the titanium abutments (IAC). The single crown/abutment restorations<br />
show custom emergence profiles.
90 <strong>EDI</strong><br />
Clinical Science<br />
Fig. 13 Template for inserting single crowns. Note the green<br />
implant analogues with the sloping shoulder, the 3-mm<br />
posts reducing the required space at crest level and the<br />
custom emergence profile of the IACs.<br />
Fig. 15 Control one year after insertion of the upper IACs.<br />
Fig. 17 Extraoral view of the smile line.<br />
The advantages of using latest-generation poly -<br />
ceramic materials are linked to their high elasticity,<br />
resistance to traction and above all to light-curing,<br />
which prevents oxidation of the post, keeping its<br />
integrity and preserving the locking-taper connection.<br />
The abutment/crown units produced are characterized<br />
by individual emergence profiles and perfect<br />
adaptation of the polyceramic material to the<br />
titanium abutment at the marginal gap level. All<br />
crowns produced in this way were placed on the cast,<br />
and an oral repositioning stent and a taping jig were<br />
produced (Fig. 13).<br />
As each Integrated Abutment Crown was completed,<br />
it was inserted with its post fitting the into the<br />
connector socket of the implant and activated by<br />
pressing onto the crown itself (Fig. 14).<br />
The custom emergence profiles of each crown<br />
ensured that the surrounding gum was shaped such<br />
as to produce a natural and healthy-looking gingival<br />
contour (Figs. 15 to 18).<br />
Fig. 14 Insertion of an Integrated Abutment Crown (IAC).<br />
The screwless connection without index allows the post to<br />
be inserted into the connecting socket with simple rotational<br />
movements and is then tapped into position.<br />
Fig. 16 Note the long-term preservation of the gingival contour.<br />
Fig. 18 Control ten months after insertion of the lower IACs.<br />
Materials and methods<br />
Bicon dental implants were used for the reconstruction<br />
of the case, combined with Ceramage poly -<br />
ceramic materials (Shofu Inc., Kyoto, Japan) for the<br />
restoration.<br />
Bicon implants stand out on account of their special<br />
macrostructure that is characterized by a rootshaped<br />
design with wide fins called plateaus, by a<br />
sloping shoulder and by a connecting socket that<br />
accommodates the abutment post by means of a<br />
locking-taper connection [7].<br />
The plateaus are of particular importance for biomechanical<br />
performance, allowing Short Implants<br />
with a wide diameter to be used in any position in the<br />
oral cavity. They are inserted into the osteotomy, prepared<br />
using atraumatic drills rotating at 50 rpm, using<br />
mechanical pressure. The countless microretentions<br />
created by the fin edges on the walls of the osteotomy<br />
ensure primary stability of the implant. Further-
<strong>EDI</strong> 91<br />
Clinical Science<br />
more, the wide spaces between the plateaus avoid<br />
vertical compression of the bone walls and rapidly<br />
collect the coagulated blood, allowing rapid bone formation<br />
without the classic macrophage and osteoclast<br />
processes of bone resorption. Well-defined bone<br />
is formed, with Haversian canals and blood vessels<br />
that enable continuous bone remodelling around the<br />
implant/bone interface. This ensures implant stability<br />
in any situation involving biomechanical stimulus [8].<br />
The sloping shoulder is vitally important for preserving<br />
crestal bone after implant osseointegration<br />
and for implant function. The Bicon implant design<br />
offers platform switching with a neck that converges<br />
from the widest diameter of the first plateau to 2<br />
or 3 mm towards the crestal zone (converting crest<br />
module). In the case presented here we used 5 mm<br />
diameter implants, but the space required at the crestal<br />
level is only 3 mm. This ensures bone augmentation<br />
above the neck, not least because, during the<br />
first surgical stage, the implant is positioned at least<br />
1 mm below the crest. This allows structures such<br />
as the crestal bone, periosteum and epithelium to<br />
grow around the hemispherical base of the abutment,<br />
and to give sufficient space for papilla maintenance<br />
and/or growth.<br />
Another factor important for long-term crestal<br />
bone stability is the bacterial seal at the implantabutment<br />
interface. If crestal bone maintenance and<br />
the formation of papillae can only be achieved by an<br />
implant placed in a subcrestal position and platformswitching<br />
at the level of the implant neck, it is also<br />
true that this situation can only be achieved if the<br />
gap is hermetically sealed against bacterial infiltration.<br />
Without this feature, the placement of a subcrestal<br />
implant without a bacterial seal would result in<br />
a rapid spread of pathogens around vital structures<br />
such as the crestal bone, periosteum and epithelium.<br />
The result would be bone resorption to well below<br />
the original crestal bone level.<br />
Bicon’s locking taper is indispensable for ensuring<br />
maintenance of the crestal bone level around an<br />
implant with a convergent sloping shoulder placed<br />
subcrestally [9].<br />
The locking taper is an extremely precise connection<br />
formed by contact welding of two surfaces of<br />
the same material brought into close contact. In this<br />
way, the oxidation layers that have formed on the<br />
abutment post and on the implant connector surface<br />
are removed [10, 11].<br />
The prosthetic components (one-piece titanium<br />
abutments made from the same surgical-grade titanium<br />
alloy as the implants) ensure maximum mechanical<br />
resistance and optimum biocompatibility. The subgingival<br />
hemispheric geometry is ideal for ensuring<br />
the stability of the peri-implant connective tissues.<br />
bionic sticky granules<br />
Ingenious: Simple handling<br />
and accelerated osteoconduction<br />
for long-term volume<br />
preservation.<br />
Order your free test sample<br />
over the internet!<br />
Degradable Solutions AG<br />
Wagistrasse 23<br />
CH-8952 Schlieren<br />
Phone: +41 43 433 62 60<br />
www.degradable.ch<br />
dental@degradable.ch<br />
easy-graft ® CRYSTAL
92 <strong>EDI</strong><br />
Clinical Science<br />
The abutments are connected to the implant well by<br />
a post 2 or 3 mm in diameter. Implants 3.5 and 4 mm in<br />
diameter are suitable for 2-mm posts, while 4.5, 5 or<br />
6 mm diameter implants match abutments with 3-mm<br />
posts. Abutments with 2- and 3-mm posts have diameters<br />
or emergence profiles of 3.5/4.0/5.0/6.5 mm, suitable<br />
for recreating natural anatomical gum shapes.<br />
Abutment diameters are therefore independent of<br />
implant diameters. Each implant may host four different<br />
abutment emergence profiles. The different<br />
emergence profiles start from the 2- or 3-mm posts,<br />
placed at the crestal level.<br />
The geometry of the abutments provides for platform<br />
switching even at a prosthetic level, which is<br />
of vital importance for the connecting tissue and<br />
epithelial layer.<br />
The supraperiosteal space involved in the shift<br />
from the connecting-post diameter (2-3 mm) to the<br />
diameter of the abutment hemisphere (5-6.5 mm)<br />
allows thicker and more dense connecting tissue to<br />
form, resulting in optimal preservation of the papilla.<br />
In the case described here, all abutments had 3-mm<br />
posts, as they had to connect to the 3-mm connector<br />
sockets of the 5.0 x 6.0 mm implants. Heights, inclinations<br />
and hemisphere diameters are selected in<br />
the laboratory in accordance with the positions of<br />
the implants and the mesiodistal space available.<br />
The space created by the bone distraction in the<br />
anterior zone is filled with Pure Phase Beta-Tricalcium<br />
Phosphate (SynthoGraft). This completely synthetic<br />
bone grafting material eliminates the uncertainty<br />
and the risks associated with materials derived from<br />
humans or animals. SynthoGraft has a unique structure<br />
(two variants: 50-500 μm and 500-1000 μm),<br />
which ensures stability during handling, while its<br />
microporosity allows rapid vascularization with subsequent<br />
resorption and replacement with autologous<br />
bone [12].<br />
The material used for IAC preparation is Ceramage,<br />
a microceramic composite system containing 73 per<br />
cent of microfine ceramic filler particles – Progressive<br />
Finely Structured (PFS) filler and nanohybrid fillers –<br />
supported by a polymer matrix. This strengthens the<br />
homogenous structure and makes the material similar<br />
to a ceramic material, to be used both for posterior<br />
and anterior restorations with metal or metal-free<br />
frameworks.<br />
Discussion<br />
This case highlights the predictability of Short Implants<br />
and, in particular, Ultra Short Implants. Both<br />
have been widely described in the literature [13-15]<br />
and numerous long-term studies have demonstrated<br />
their suitability over time [16, 17].<br />
The 6.0-mm length used is classified as an ultrashort<br />
implant. Its unique geometry gives the implant<br />
a bone stability capable of supporting a crown/<br />
implant ratio that can be above the 4 : 1 ratio [18]. This<br />
concept overturns the classic demand for a crown/<br />
im plant ratio of 1 : 1. According to what we know<br />
today about the importance of implant design for<br />
the function of the crown/implant complex [19-22],<br />
the contact area (BIC) of the implant surface with the<br />
recipient bone is a decisive factor, as are the diameter<br />
and the macrogeometry of the fixture – not necessarily<br />
its length [23-25].<br />
Conclusion<br />
Modern oral implantology must rely on exact biomechanical<br />
and histomorphological criteria to<br />
obtain long-term results satisfactory to clinicians<br />
and patients [26].<br />
Recent studies underscore the importance of<br />
improving implant macrostructures, particularly<br />
around the implant neck [27]. The crestal bone needs<br />
space to support the surrounding structures. Only<br />
then can the gingival architecture and papillae be<br />
maintained in the long term [28]. Simple platform<br />
switching at the abutment level is no guarantee of<br />
space, while platform switching at the implant neck<br />
level is crucial. The implant used in our clinical case<br />
has fulfilled both criteria for over 25 years (Fig. 19).<br />
The Integrated Abutment Crown (IAC) technique<br />
further improves the condition of the soft tissues,<br />
eliminating the need for cementation and avoiding<br />
any misfits between the border of the crown and<br />
the preparation of the abutment. Furthermore, the<br />
custom emergence profile ensures proper gingival<br />
anatomy [29,30].<br />
A list of references can be found at www.teamwork-media.de<br />
Contact address<br />
Professor Mauro Marincola<br />
Studio Dentistico Camardent<br />
Via dei Gracchi 285 . 00192 Roma . Italy<br />
Phone: +39 06 3215056<br />
mauromarincola@unicartagena.edu.co<br />
Fig. 19<br />
Radiological<br />
control at<br />
24 months<br />
showing the<br />
preserved crest<br />
around the platform-switched<br />
implant shoulder<br />
and the hemispherical<br />
base of<br />
the abutment.
Sie kennen den<br />
richtigen Zeitpunkt.<br />
Ermittlung des optimalen<br />
Belastungszeitpunktes basierend<br />
auf standardisierten ISQ-Werten<br />
Frühzeitige Warnungen durch<br />
die kontinuierliche Kontrolle der<br />
Osseointegration<br />
Richtig?<br />
Mit Osstell ISQ bestimmen Sie<br />
einfach und schnell den optimalen<br />
Belastungszeitpunkt von Implantaten<br />
- bei Sofort- und auch bei Spätbelastungen.<br />
Auf der Grundlage von exakten<br />
Messwerten können Sie den Grad der Osseointegration<br />
vom Zeitpunkt der Implantation bis zur endgültigen<br />
Versorgung verfolgen. Vor allem die Behandlung von<br />
Risikopatienten wird damit sicherer und besser einschätz-<br />
Einfache und schnelle Messung<br />
der Implantatstabilität<br />
bar. Osstell ISQ ist das einzige objektive Qualitätssicherungssytem,<br />
das Sie rechtzeitig warnt, wenn Implantate<br />
nicht wie erwartet osseointegrieren. Objektive ISQ-Werte<br />
erleichtern es Ihnen, Patienten und Kollegen Behandlungspläne<br />
und Einheilungszeiten zu<br />
erklären. Sie sind erfahren und treffen<br />
die richtigen Entscheidungen –<br />
Osstell ISQ bringt Ihnen und Ihren<br />
Patienten nun eine neue Sicherheit.<br />
www.osstell.com Die objektive Messung der Implantatstabilität<br />
Besuchen Sie den Osstell Stand auf der Gemeinsamen DGI Jahrestagung der Landesverbände Bayern<br />
und Baden-Württemberg am 27. und 28. Mai 2011 in Ulm.
94 <strong>EDI</strong><br />
Business & Events<br />
Innovations by Dentsply Friadent at IDS 2011<br />
Detailed solutions<br />
“from root to crown”<br />
At IDS 2011, Dentsply Friadent will present a number of additions to its range of implant products. In the following statement,<br />
Marketing Director Birgit Dillmann is talking about these new solutions that blend in perfectly with the company’s overall<br />
concept and further reduce chairside treatment times, thus increasing patient satisfaction.<br />
For Dentsply Friadent, providing complete solutions<br />
“from root to crown” means providing users with a<br />
comprehensive product portfolio for every step of<br />
their dental implant procedures. Beginning with precisely<br />
timed digital planning and a stable base for<br />
implantation to the implantation itself and right up<br />
to the aesthetic completion of the treatment –<br />
Dentsply Friadent offers detailed implant solutions<br />
for every individual case.<br />
At the same time, we strive to develop all our treatment<br />
concepts towards TissueCare, because the only<br />
secure basis of effective treatment is the stability of<br />
hard and soft tissues over the long term.<br />
We are very pleased to present new solutions at the<br />
upcoming IDS that blend in perfectly with our overall<br />
concept. Next to enhancing our implant brands with<br />
new components first and foremost, these include<br />
our digitally manufactured restorations, which stand<br />
for precision and for simplifying daily routines in your<br />
practice. With our now commonly organized central<br />
Compartis Scanning and Design Service for custom<br />
abutments and CAD/CAM superstructures, you can<br />
order individual implant prosthetics for your patients<br />
even faster and get competent advice at any time.<br />
Apropos of digital solutions: our Guided Surgery<br />
System will help you shorten chairside treatment<br />
times and increase patient satisfaction. Because with<br />
ExpertEase, the temporary denture can already be<br />
fabricated before the template-guided implant<br />
placement so that the denture can be incorporated<br />
directly, without delay.<br />
Digital solutions – for many practitioners they have<br />
become indispensable, so they are an important part<br />
of our portfolio. As a solution supplier and a partner<br />
of your successful practice, however, we provide you<br />
with much more: Our individual implant systems, for<br />
outstanding primary stability in all bone qualities<br />
and enduring tissue stability. Our prosthetic concepts,<br />
for aesthetics and perfect functionality. And<br />
ultimately, we will help you become a brand in your<br />
region – with our stepps customer program, a comprehensive<br />
program for marketing and office/laboratory<br />
management.<br />
We would be delighted to tell you more about all<br />
this and show you the full range of our offering.<br />
Come and meet us at IDS – we are looking forward to<br />
seeing you!<br />
Birgit Dillmann
96 <strong>EDI</strong><br />
Business & Events<br />
An interview with Frank Bartsch, Trade Marketing Manager at Carestream Health<br />
“Innovation is our focus<br />
at the IDS 2011“<br />
In the run-up to IDS 2011, <strong>EDI</strong> Journal talked to Frank<br />
Bartsch, Trade Marketing Manager at Carestream<br />
Health, about the new business division Carestream<br />
Dental and the forthcoming product launches of the<br />
company at the IDS.<br />
What can visitors to the IDS expect from Carestream?<br />
Plenty, of that you can be sure! In Cologne, we will<br />
be presenting a number of surprises in the field of<br />
X-ray imaging. We have also increased our floor space<br />
considerably compared to IDS 2009, and we have<br />
added some attractive features. For example, our visitors<br />
can look forward to our “Innovation Room” that<br />
showcases our highlighted exhibits in detail.<br />
The focus of our presentation will of course be on<br />
the 9000 series imaging range. In late 2007, this<br />
range established a new class in X-ray imaging, which<br />
has lost nothing in terms of cutting-edge technology<br />
and fascination. In 2009 we had already updated<br />
the 9000 3D model by adding the Stitching Function.<br />
Now we have extended the product range and will<br />
present the CS 9300, the market leader’s “big brother”<br />
in focus-field imaging. The CS 9300 not only combines<br />
panorama and 3D technology, but also offers a<br />
number of innovative features, such as free choice of<br />
viewing field (5 x 5 cm up to 17 x 13,5 cm) – “Flexy-Fieldof-View”<br />
– with excellent resolution, simple-to-use<br />
software and imaging details second to none.<br />
And what about the cost?<br />
The CS 9300 model offers an excellent price/performance<br />
ratio. Model calculations have shown that the<br />
investment pays off already after a short time based<br />
on average use. Together with our business partners<br />
we have arranged for attractive financing packages<br />
that will facilitate economical use from the outset.<br />
As the only single-source manufacturer worldwide for<br />
the entire product range from films to 3D imaging, you<br />
can provide customized solutions for all X-ray imaging<br />
requirements. What can smaller dental practices expect?<br />
Here we are looking primarily at two models we<br />
are very proud of. The CS 7600 is an innovative intra-<br />
oral scanner that defines new benchmarks in terms<br />
of speed, efficiency and product features. To mention<br />
just one of the benefits: images are available within<br />
five seconds. And the CS 1600 intraoral camera sets<br />
new standards in the detection of caries. This addition<br />
to our highly successful 1500 camera allows the<br />
detection of caries at a very early stage by employing<br />
the patented FIRE technology.<br />
Another interesting aspect is your recently created new<br />
business division, Carestream Dental. What can you tell<br />
us about this?<br />
This development reflects the growing importance<br />
of the dental business. We intend to focus more heavily<br />
on this field in the future and to align our service<br />
with the specific needs of our customers. Therefore we<br />
decided to split the Carestream Health brand into a<br />
Carestream Dental and a Carestream Medical division.<br />
Can you give us a glimpse of the future?<br />
The name Carestream already stands for a wealth<br />
of innovation and first-class products with excellent<br />
price/performance ratios. This will continue to be the<br />
case in the future. We want to ensure that our customers<br />
benefit from our X-ray imaging expertise over<br />
the long term. We provide unbeatable imaging<br />
results allowing for optimum diagnostics and efficient<br />
treatment planning, which in turn result in considerable<br />
time savings and high levels of efficiency.<br />
This is true today – and it will be true tomorrow!<br />
Mr. Bartsch, thank you for this interview. SIS
Tixos is a porous surface<br />
characterized by<br />
interconnected cavities,<br />
with predetermined<br />
geometry, that enhance<br />
fast bone formation*.<br />
* References available upon request.<br />
L E A D E<br />
R<br />
I T A L I A<br />
The Laser Microfused Titanium Surface<br />
by LEADER IMPLANTS<br />
Faster Bone Growth<br />
inside the cavities<br />
of the<br />
microfused titanium<br />
surface<br />
EXTRAORDINARY BONE GROWTH<br />
INSIDE THE IMPLANT CONCAVITIES<br />
lasermade<br />
titanium<br />
bone<br />
100 μm<br />
COLOGNE<br />
22-26 MARCH<br />
Visit us!<br />
LEADER ITALIA s.r.l.<br />
Hall 10.2<br />
Via Aquileja, 49 - 20092 Cinisello Balsamo (MI) ITALY Stand U 19<br />
ph. +39 02 618651 - fax +39 02 61290676 - www.leaderitalia.it - export@leaderitalia.it
98 <strong>EDI</strong><br />
Business & Events<br />
An interview with Professor Daniel Buser and Professor Mariano Sanz<br />
on the International Osteology Symposium in Cannes<br />
Risk factors and complications<br />
in regeneration<br />
The next International Osteology Symposium is being held from 14–16 April 2011 in Cannes. What will the symposium be offering<br />
to practitioners and scientists? The international chairmen of the symposium, Professors Mariano Sanz and Daniel Buser,<br />
respond to questions in an interview.<br />
Another International Osteology Symposium is now<br />
being held after four years. What are the main topics<br />
of interest?<br />
Professor Buser: We have chosen the main topics<br />
“Clinical Excellence, Risk Factors and Complications”<br />
for the symposium. Nowadays bone regeneration<br />
is the standard of care in implant dentistry. In uncritical<br />
cases implant surgeons can treat small to midsize<br />
defects such as apical fenestration or crestal<br />
dehiscence defects with high predictability to<br />
achieve successful outcomes. It is very important,<br />
that the clinician can correctly assess risk factors<br />
and integrate them into treatment planning in more<br />
complex cases.<br />
Professor Sanz: Our patients not only want functional<br />
results, they also want beautiful smiles. The<br />
right proportions between beautiful teeth and gums<br />
is what makes a nice smile and that is precisely what<br />
our patients are looking forward to. We therefore<br />
need to balance the importance of risk factors and<br />
complications with techniques aimed to achieve aesthetic<br />
results. This is the reason why the field of softtissue<br />
management has gained an important impetus<br />
in the last few years, both in implantology and<br />
periodontology. At Osteology in Cannes we will be<br />
discussing new therapeutic approaches, new biomaterials<br />
and improved surgical techniques to augment<br />
both hard and soft tissues, both around teeth and<br />
dental implants, in order to rebuild what oral diseases<br />
have destroyed.<br />
Just to get back to the topic of complications: What<br />
significance does the topic now have in regenerative<br />
procedures?<br />
Professor Buser: As the number of inserted im -<br />
plants has significantly increased in the past ten years,<br />
the number of complications such as peri-implantitis<br />
also rises. Therefore, a section of the programme in<br />
Cannes deals exclusively with the topic of periimplantitis.<br />
Well-known experts will be reporting on<br />
the prevalence, risk factors and pathogenesis of the<br />
disease. They will demonstrate what surgical and<br />
non-surgical therapies are indicated for what cases<br />
and they will show when regenerative treatments<br />
promise success.<br />
Professor Sanz: Besides the occurrence of periimplant<br />
infections and complications, we will discuss<br />
other multiple sources of complications and most<br />
importantly, how to diagnose and how to treat them.<br />
Complex therapeutic procedures, such as soft tissue<br />
augmentation, guided bone regeneration or sinus<br />
floor augmentation will be discussed in the context<br />
of the treatment of complicated cases. Special<br />
emphasis will be placed in how to prevent complications<br />
and how to achieve the best possible outcomes.<br />
Professor Daniel<br />
Buser (left) and<br />
Professor Mariano<br />
Sanz will be chairing<br />
the International<br />
Osteology<br />
Symposium<br />
in Cannes.
The Osteology symposia are well-known for their high<br />
scientific standard. Is Osteology in Cannes sufficiently<br />
practice-oriented?<br />
Professor Sanz: Absolutely! The motto of the Osteology<br />
Foundation is “Linking Science with Practice”,<br />
and we consistently make this happen in our symposia.<br />
Science provides the basis for new therapeutic<br />
concepts, but the wide usage of these concepts in<br />
practice cannot be widespread until this treatment<br />
option or product has been sufficiently tested and<br />
backed up by scientific data. The presentation of<br />
new scientific data has, therefore, always taken an<br />
important place at the Osteology symposia programmes.<br />
We strongly believe that only well<br />
informed practitioners would be able to provide<br />
optimum treatments for their patients. But we do<br />
not overlook the practical applications of current<br />
therapies. On the pre-congress day there is a large<br />
selection of practical hands-on courses and theoretical<br />
workshops. In this Osteology Symposium we<br />
have also organized an interactive clinical forum in<br />
which top-class experts and the audience will be<br />
discussing exciting complex cases.<br />
����������������������������<br />
�����������������������������������������<br />
���������������������������<br />
������������������������������������������������<br />
�����������������������������<br />
��������������������������������������������<br />
��������������������������<br />
��������������������������<br />
�����������������<br />
������������������<br />
����������<br />
���<br />
����������<br />
�����������������������<br />
�������<br />
���<br />
������������������<br />
��������������������<br />
������������������������������<br />
� � �<br />
�������� �������� ��������<br />
�������� �������� �������� ��������<br />
�������� �������� ��������<br />
�������� �������� ��������<br />
���<br />
���<br />
��<br />
���<br />
���<br />
��<br />
�������� ��������<br />
<strong>EDI</strong> 99<br />
Business & Events<br />
What else is on the programme in Cannes?<br />
Professor Buser: The programme embraces a broad<br />
selection of indications in implantology and periodontology.<br />
We will be discussing whether new findings<br />
cast doubt upon well established treatment<br />
concepts, and what new therapies and products<br />
could be reliably used in daily practice in the future.<br />
As ever at the Osteology symposia, we not only<br />
offer participants a top-class, exciting scientific programme,<br />
but also an exceptional atmosphere and a<br />
fascinating social programme. Osteology in Cannes is<br />
making another guest appearance on one of Europe’s<br />
most beautiful coasts. This guarantees inspiring days<br />
at the congress. Our colleagues should not miss that<br />
highlight in 2011!<br />
Thank you very much for this interview.<br />
Dr Birgit Wenz, Lucerne<br />
More information<br />
Osteology Foundation<br />
www.osteology-cannes.org<br />
Brilliant in Any Language<br />
����������������������������������������������������<br />
�������������� �������������� �������������� �������������� �������������� �������������� �������������� ��������������<br />
�������������<br />
��<br />
��<br />
��<br />
��<br />
��<br />
��<br />
��<br />
��<br />
��<br />
��<br />
��<br />
��<br />
��<br />
��<br />
���������������������<br />
����������<br />
���<br />
���<br />
���<br />
���<br />
����������<br />
���<br />
����������������<br />
������������<br />
��<br />
��<br />
��<br />
��<br />
��<br />
��<br />
�����<br />
Contact Aseptico for more Information Infor Information<br />
int@aseptico.com ������������������������������������������<br />
����������������������������������������������������������������������<br />
�������������������������������������������������������������������������������������������������������
100 <strong>EDI</strong><br />
Business & Events<br />
The 1st MIS Global Conference is an exclusive international<br />
event in the Osseointegration world in 2011,<br />
where science and clinical expertise meet on the<br />
background of warm white powdery beaches and<br />
turquoise clear waters.<br />
During the conference’s three exciting days, participants<br />
are expected to broaden their horizons<br />
and enhance their knowledge in implant dentistry<br />
through lectures by highly qualified and respected<br />
first class opinion leaders and pioneering researchers<br />
MIS Global Conference,<br />
Cancun, Mexico, 19 to 21 May 2011<br />
360° Implantology<br />
The first MIS Global Conference entitled „360° Implantology” will be<br />
held in the magnificent Hilton Cancun Golf & Spa Resort in Mexico<br />
from 19 to 21 May 2011. 360° Implantology represents MIS’s vision<br />
based on a deep understanding of all professionals’ needs in implant<br />
dentistry that will be presented in an exceptional, elegant and<br />
sophisticated event organization.<br />
and practitioners that will present the latest innovations<br />
and advances in the field along with a wide<br />
range of compelling topics. MIS’s latest virtual guided<br />
implant system M-Guide, an innovative new MIS<br />
implant system, and the innovative graft material<br />
Bond Bone are just a few of the advances that will be<br />
presented during the conference. Participants can<br />
also extend their knowledge and experience by<br />
attending a variety of influential and beneficial workshops<br />
and hands-on sessions.<br />
More information<br />
MIS Implants Technologies Ltd<br />
www.mis-implants.com/cancun
<strong>EDI</strong> 101<br />
Business & Events<br />
Thommen Connecting Science Workshop<br />
during the International Osteology Symposium<br />
in Cannes<br />
Risk factor bone<br />
Under the topic of “Risk factor bone” Thommen Medical will hold an<br />
exclusive Connecting Science Workshop Session taking place on 14 April<br />
2011, 9:00 am – 12:30 pm, during the course of the Osteology Symposium<br />
in Cannes. The participants can expect an outstanding scientific program<br />
with excellent speakers and interactive round panel discussions. The mod-<br />
erator of the program will be Dr Ueli Grunder from Zurich, Switzerland.<br />
The workshop will focus on different<br />
aspects related to weak bone<br />
quality: meaning either insufficient<br />
bone volume or bone types<br />
III or IV.<br />
Dr Michaela Kneissel from the<br />
Novartis Institute of Biomedical<br />
Research will focus on the biologic<br />
background of maxillofacial<br />
bone regeneration and the underlying<br />
mechanisms in different<br />
bone types. Furthermore, various<br />
clinical aspects of diseases affecting<br />
bone regeneration and comedication<br />
will be addressed.<br />
Professor Markus Huerzeler will<br />
present current approaches in<br />
socket preservation in the aesthetic<br />
zone and some new preservation<br />
techniques for the buccal wall.<br />
PD Dr Dr Dennis Rohner from<br />
the Cranio-Maxillofacial Center in<br />
Aarau will summarize the treatment<br />
options for patients with<br />
poor bone quality, underlying diseases<br />
or otherwise disturbed<br />
bone regeneration. The role and<br />
function of a superhydrophilic<br />
implant surface (Inicell) will be<br />
demonstrated and data from a<br />
clinical study on reduced healing<br />
time in patients with bone quality<br />
III and IV presented. Future<br />
developments in implant surface<br />
modifications and co-medication<br />
will be addressed by Dr Michaela<br />
Kneissel. Based on a newly developed<br />
ostoporotic rat model, several<br />
new approaches will be presented.<br />
Additional<br />
program highlight<br />
Thommen Medical will welcome<br />
all Osteology participants to a further<br />
Thommen Session that takes<br />
place within the official Osteology<br />
Implant Forum on Thursday,<br />
14 April 2011 at 1:00 pm. Here Dr Konrad<br />
Meyenberg, Switzerland, will<br />
hold an exclusive lecture on the<br />
topic “Smart Design – the basis for<br />
biological, technical and esthetic<br />
success”.<br />
More information<br />
and registration<br />
Thommen Medical AG<br />
Hauptstrasse 26d<br />
4437 Waldenburg<br />
Switzerland<br />
www.osteology-cannes.org<br />
www.thommenmedical.com/events<br />
3D Composites<br />
Natural Shading & Shaping<br />
By Ulf Krueger-Janson<br />
Dentist Ulf Krueger-Janson is considered one of the<br />
foremost global experts on functional and aesthetic<br />
chairside composite techniques.<br />
His new book fi rst invites the reader to train his or her<br />
senses to consciously take in and to reproduce shapes,<br />
contours, but also the shades of various teeth. Once<br />
the senses have become working, the implementation<br />
phase follows. The book contains practical instructions<br />
for an uncomplicated build-up as well as tips and<br />
tricks for the correct handling of appropriate materials<br />
and tools. Finally, the reader will have a chance to<br />
follow the author and his techniques as exemplifi e d<br />
by selected patient cases, organized by the different<br />
restoration types and locations.<br />
The book comprises eight chapters with easy-to-follow<br />
didactics, a stunning layout and brilliant illustrations. A<br />
book that inspires, teaches and motivates – a “must<br />
have” for every ambitious practitioner.<br />
€ 178<br />
(including VAT)<br />
264 pages, approx. 1300 illustrations<br />
ISBN: 978-3-932599-29-3<br />
REF 9028<br />
Visit our bookshop or order via<br />
Phone +49 8243 9692-16<br />
g.konuk@teamwork-media.de<br />
NEW<br />
www.teamwork-bookshop.de
102 <strong>EDI</strong><br />
Business & Events<br />
European market leader in medical<br />
B2C communication celebrates its 5,000th installation<br />
TV-Wartezimmer’s<br />
market share on the rise<br />
Today’s health-conscious and responsible patients expect comprehensive information on illnesses, alternative treatment methods,<br />
prevention options and healing prospects. They are willing to invest in their personal health and well-being. This makes it<br />
essential for doctors and clinics to communicate their profiles, services and qualifications in an efficient and sophisticated<br />
manner. Patients want this information and are eager to learn how useful investments into their own health can be –<br />
for example in terms of prevention efforts.<br />
Efficient contemporary communication platforms for<br />
offices and clinics are on their way ahead. “Only innovative<br />
solutions that focus on the doctors’ specific<br />
needs will prevail”, says Markus Spamer, founder and<br />
CEO of TV-Wartezimmer. The 5,000 systems his company<br />
has sold bear witness to the truth of his statement.<br />
“TV-Wartezimmer has proven to be of multiple<br />
benefit in medical waiting areas.” Patients are<br />
informed and entertained by attractive content, while<br />
at the same time TV-Wartezimmer fills an important<br />
purpose for doctors by providing patients with relevant<br />
medical information, ahead of treatment.<br />
Markus Spamer founded TV-Wartezimmer in 2003<br />
after learning that many professionals have problems<br />
communicating with their customers in a way<br />
that makes them act on the message they were<br />
supposed to receive. Since then, TV-Wartezimmer has<br />
established itself as the European market leader in<br />
audio-visual doctor-patient communication solutions.<br />
Total revenue for 2009 was 7.5 million euros, and the<br />
company employs 40 people at its headquarters in<br />
Freising near Munich. More than 100 sales and service<br />
partners complement the company’s team.<br />
TV-Wartezimmer does not focus on healthcare topics<br />
alone. It includes such diverse matters as wildlife<br />
and travel documentaries, news, regional weather<br />
forecasts, gossip news, comic strips and much more,<br />
all which is continuously updated, some of it at hourly<br />
intervals. But the core of TV-Wartezimmer is the wide<br />
range of films, offered for free use, that show all the<br />
additional services, therapies or prevention programs<br />
that a practice can offer. Each topic presents itself<br />
in a contemporary, three-dimensional look and uses<br />
a language that explains even complex procedures in<br />
terms the medical layperson can understand.<br />
TV-Wartezimmer’s production<br />
company, which has won multiple<br />
international awards, has produced<br />
more than 400 films of<br />
this kind so far – in HD quality.<br />
TV-Wartezimmer’s customers purchase<br />
a comprehensive user license<br />
to these films that includes their<br />
use on websites or iPad offerings. The company<br />
provides continuous content updates, personalized<br />
customer support, the complete hardware including<br />
42-inch state-of-the-art displays and a free installation<br />
service. The license fee, payable monthly, covers<br />
all these and further features. There are no hidden<br />
fees or additional costs. “This is one of the reasons<br />
why we lead the market today”, says Markus Spamer.<br />
“Our understanding of good service is that all product<br />
features should always be included.”<br />
The company itself is enthusiastic about its product<br />
– and so are its customers: When TV-Wartezimmer<br />
asked for letters of reference, the company was<br />
overwhelmed by the flood of responses. About 200 of<br />
them were published in a book presented at M<strong>EDI</strong>CA,<br />
the world’s largest medical fair, in 2010. With such<br />
great support, TV-Wartezimmer looks optimistically<br />
into the future and plans to expand to additional<br />
European countries.<br />
More information<br />
TV-Wartezimmer<br />
Raiffeisenstraße 31<br />
85356 Freising<br />
Germany<br />
info@TV-Wartezimmer.de<br />
www.TV-Wartezimmer.de
H&H Webranking survey<br />
Nobel Biocare’s corporate website has been ranked<br />
the fifth best website in Switzerland by Hallvarsson &<br />
Hallvarsson. It is thus hot on the heels of the top 4<br />
websites of Swisscom, UBS, CS, and Georg Fischer.<br />
Nobel Biocare was able to improve its position from<br />
last year’s sixth place.<br />
The H&H Webranking survey is the most in-depth<br />
analysis of online financial and corporate communication<br />
in Europe. It represents a useful instrument<br />
not only for measuring the effectiveness of a company’s<br />
online communication but also to compare it<br />
with competitors on a national and international<br />
level. H&H conducts an analysis of corporate web-<br />
<strong>EDI</strong> 103<br />
Business & Events<br />
Nobel Biocare’s website breaks top 5<br />
According to the annual Hallvarsson & Hallvarsson (H&H) study, Nobel Biocare’s corporate website ranks fifth<br />
among 48 Swiss companies, moving up one position from last year’s sixth place.<br />
Cologne, 22-26.03.2011<br />
Visit us!<br />
Hall 4.1 Stand C088- D089<br />
sites in English through an evaluation protocol composed<br />
of 127 criteria. The results are based on questionnaire<br />
feedback from 571 analysts, journalists and<br />
investors throughout Europe.<br />
The Nobel Biocare corporate website recently also<br />
ranked fourth best website in the webranking car-<br />
ried out by Bilanz, a leading Swiss<br />
business magazine. The corporate<br />
website was launched in January<br />
2009 and has been continuously<br />
enhanced since, as part of a series<br />
of initiatives to improve Nobel Bio -<br />
care’s online presence.<br />
www.omniaspa.eu<br />
More information<br />
Nobel Biocare Management AG<br />
P.O. Box<br />
8058 Zurich-Airport<br />
Switzerland<br />
www.nobelbiocare.com<br />
Since our beginnings, we have always been focused on<br />
quality and innovation toward the battle against<br />
cross - contamination and infections.<br />
In the last 20 years, we have ensured safety and protection to you and your patients,<br />
with advanced and reliable products. Tools that represent the ideal solution for who is<br />
operating in dentistry, implantology/oral surgery and general surgery.<br />
With Omnia sure to be safe.<br />
OMNIA S.p.A.<br />
Via F. Delnevo, 190 - 43036 Fidenza (PR) Italy - Tel. +39 0524 527453 - Fax +39 0524 525230<br />
VAT IT 01711860344 - R.E.A. PR 173685 - Company capital € 200.000,00<br />
by
104 <strong>EDI</strong><br />
Product Reports<br />
Alpha-Bio’s Graft by Alpha-Bio Tec<br />
Safety and reliability in dental<br />
bone and tissue regeneration<br />
Alpha-Bio Tec has launched a new line of bone and tissue regeneration products – Alpha-Bio’s Graft.<br />
It offers simple, safe and reliable solutions for a wide range of clinical indications.<br />
Alpha-Bio’s Graft product line offers a variety of highquality<br />
bone and tissue regeneration products to suit<br />
all dental requirements and indications – from the<br />
simplest extraction socket filling to the most complicated<br />
bone augmentation. It includes Natural Bovine<br />
Bone, Synthetic Resorbable Bone, Collagen Membrane<br />
and Collagen Fleece. Alpha-Bio’s Graft products<br />
were developed in order to provide simple, easy to<br />
handle bone and tissue regeneration products that<br />
are highly reliable and yield outstanding, predictable<br />
results. During the regeneration process, each product<br />
in the Alpha-Bio’s Graft line becomes an integral<br />
part of the newly forming bone framework. All products<br />
have been extensively clinically tested. They<br />
are produced under cleanroom conditions and with<br />
accordance to the highest quality and safety standards.<br />
Alpha-Bio’s Graft products are CE-marked in<br />
accordance with the Council Directive 93/42/EEC and<br />
Amendment 2007/47/EC.<br />
Alpha-Bio’s Graft Natural Bovine Bone<br />
Alpha-Bio’s Graft Natural Bovine Bone is a highly reliable<br />
and dimensionally stable, purified grafting<br />
material. The mineral composition, three dimensional<br />
structure and the physico-chemical and biological<br />
properties of natural bovine bone are very similar to<br />
those of human bone. The special manufacturing<br />
process involves high-temperature heating. The<br />
process removes all organic components and eliminates<br />
all potential immunological reactions, making<br />
Alpha-Bio’s Graft Natural Bovine Bone 100 per cent<br />
BSE-safe and 100 per cent protein-free. The product<br />
fulfils all requirements of the Annex II.4 of the Directive<br />
93/42/EEC and the Directive 2003/32/EC.<br />
Alpha-Bio’s Graft Synthetic Resorbable Bone<br />
Alpha-Bio’s Graft Synthetic Resorbable Bone is an<br />
innovative, safe, reliable and fully synthetic bone<br />
graft substitute with improved controlled resorption<br />
properties and pleasing handling characteristics. The<br />
homogenous composition of 60 per cent hydroxyapatite<br />
(HA) and 40 per cent beta-tricalcium phosphate<br />
(ß-TCP) results in two mineral phases of activity: it<br />
supports the formation of new vital bone and maintains<br />
the volume and mechanical stability.<br />
Alpha-Bio’s Graft Collagen Membrane<br />
Alpha-Bio’s Graft Collagen Membrane provides a<br />
long-lasting adequate barrier function. Due to a special<br />
production process, the superior properties of the<br />
native pericardium are preserved, allowing to maintain<br />
the characteristics of this natural tissue.<br />
Alpha-Bio’s Graft Collagen Fleece<br />
Alpha-Bio’s Graft Collagen Fleece is pH-neutral, wetstable,<br />
haemostyptic and made out of native collagen.<br />
The potent haemostatic effect of collagen is wellknown<br />
and induced by the adhesion of platelets to<br />
the collagen fibrils. As a consequence, platelets aggregate<br />
and release coagulation factors by degranulation.<br />
This initiates the coagulation cascade resulting<br />
in haemostasis.<br />
More information<br />
Alpha-Bio Tec Ltd.<br />
7 Hatnufa St. · P.O.B 3936<br />
Petach Tikva 49510 · Israel<br />
Phone: +972 3 9291055<br />
info@alpha-bio.net · www.alpha-bio.net<br />
The product information produced here editorially is based on information<br />
provided by the manufacturer and has not been checked by the editor<br />
for its accuracy.
Join the Thommen<br />
Connecting Science TM<br />
Workshop during the<br />
Osteology in Cannes<br />
Topic: Risk factor bone<br />
Date: April 14, 2011, 09.00–12.30<br />
Language: English<br />
Price: Euro 130<br />
Speakers:<br />
Dr. Ueli Grunder, Prof. Markus Hürzeler, Dr. Michaela Kneissel, PD Dr. Dr. Dennis Rohner<br />
Program<br />
09.00 Introduction Dr. Ueli Grunder, Switzerland (Moderator)<br />
09.15 Maxillofacial bone regeneration Dr. Michaela Kneissel, USA<br />
09.45 Clinical experience with extraction sockets in the aesthetic zone:<br />
how can we preserve the volume? Prof. Markus Hürzeler, Germany<br />
10.45 Weak bone: which options do we have? PD Dr. Dr. Dennis Rohner,<br />
Switzerland<br />
11.15 Implant healing in the osteoporotic rat model Dr. Michaela Kneissel,<br />
Cambridge, USA<br />
11.45 Panel discussion Dr. Ueli Grunder, Dr. Michaela Kneissel, Prof. Markus<br />
Hürzeler, PD Dr. Dr. Dennis Rohner<br />
12.30 End of session<br />
SWISS PRECISION AND INNOVATION.<br />
www.thommenmedical.com<br />
Register now online at www.osteology-cannes.org<br />
or get more information on<br />
www.thommenmedical.com/events<br />
www.osteology-cannes.org<br />
,
106 <strong>EDI</strong><br />
Product Reports<br />
Immediate Smile by Materialise Dental<br />
Top-notch backward planning<br />
When dealing with edentulous patients, it is paramount to start at the end-point by ruling out any anatomical,<br />
functional and esthetical surprises during implant surgery. SimPlant reveals all details about a patient’s bone<br />
and soft tissue – crucial information when engaging in restorative-driven planning. Thanks to SimPlant and the<br />
Immediate Smile model, an accurate restoration can be made well in advance – seeing patients leave surgery<br />
with a carefully prepared new smile is the fruitful result of thinking and acting ahead.<br />
The Immediate Smile model plays a pioneering role<br />
on the market, in that it enhances communication<br />
with the dental lab by delivering all the necessary<br />
tools to fabricate a fixed restoration prior to surgery –<br />
a bone model, silicone soft tissue and copy of the<br />
scan prosthesis. This is interdisciplinary dentistry at<br />
its best.<br />
The bone model represents the patient’s bone<br />
anatomy and contains implant sites that correspond<br />
with the SimPlant 3D plan. The implant sites are<br />
adapted to the dimensions of the implant analogs. A<br />
clever time- and money-saving fixation system even<br />
allows for easy recuperation of implant analogs.<br />
A lifelike silicone soft tissue, which is perforated<br />
at the implant positions, represents the patient’s<br />
soft tissue, helping the lab take into account the realistic<br />
soft tissue thickness during fabrication of the<br />
restoration.<br />
A prosthesis duplicate is a copy of the scan prosthesis<br />
that fits perfectly onto the bone model allowing<br />
for accurate articulation. It also enables the lab<br />
to fabricate the restoration the way the clinician<br />
planned it. At the time of surgery, the restoration is<br />
then relined in the patient’s mouth to ensure a passive<br />
fit onto the implants.<br />
As a result, reverse planning has never been more<br />
efficient for all parties engaged in implant treatment<br />
and avoids cumbersome procedures. Clinicians don’t<br />
need to use a guide to fabricate a model, because this<br />
increases the risk of damaging the guide. Also, they<br />
can follow the same routine procedures as with all<br />
standard guided surgery procedures, and implant<br />
holders are not required.<br />
Immediate Smile makes clinicians’ businesses<br />
stand out. It’s a marketing tool which can be used<br />
to attract patients when other implant dentists do<br />
not follow a strategy of thinking ahead in terms of<br />
adopting new technology and bringing esthetics into<br />
the equation.<br />
Patients are given an accurate, predictable, esthetic<br />
and cost-efficient outcome. They won’t even have<br />
to think about taking days off at work or worry about<br />
time- and money-guzzling follow-up appointments<br />
and the drag to get there every time and on time.<br />
Overall, an Immediate Smile procedure enhances<br />
treatment acceptability because patients know they<br />
will have new teeth in no time.<br />
The Immediate Smile model is compatible with all<br />
SAFE SurgiGuide kits available on the market: Antho -<br />
gyr Guiding System (Anthogyr), Facilitate (AstraTech),<br />
Navigator (Biomet 3i), ExpertEase (Dentsply Friadent),<br />
Straumann Guided Surgery Kit (Straumann), Nobel-<br />
Guide (Nobel Biocare), Camlog Guide System (Camlog),<br />
and Zimmer Guided Surgery Instrumentation<br />
(Zimmer Dental).<br />
More information<br />
Materialise Dental NV – Headquarters<br />
Technologielaan 15<br />
3001 Leuven . Belgium<br />
Phone: +32 16 396620<br />
simplant@materialise.be<br />
www.materialisedental.com<br />
The product information produced here editorially is based on information<br />
provided by the manufacturer and has not been checked by the editor<br />
for its accuracy.
<strong>EDI</strong> 107<br />
Product News<br />
Degradable Solutions<br />
calc-i-oss crystal<br />
Product:<br />
calc-i-oss crystal<br />
Indication:<br />
Bone replacement<br />
Distribution:<br />
Degradable Solutions AG<br />
Wagistrasse 23 . 8952 Schlieren . Switzerland<br />
Phone: +41 43 433-6260<br />
dental@degradable.ch . www.degradable.ch<br />
IDS 2011 will see the first presentation worldwide of<br />
the biphasic calc-i-oss crystal by Degradable Solutions,<br />
a product consisting of 100% synthetic bone replacement<br />
particles (60% HA, 40% pure-phase ß-TCP)<br />
exhibiting a rotund, interconnected and highly porous<br />
shape. calc-i-oss crystal is the logical complement of<br />
easy-graft crystal. It is especially useful in large defects,<br />
which can be filled with calc-i-oss crystal first, then<br />
covered with easy-graft crystal. In the same manner,<br />
autologous bone or bone morphogenetic proteins (BMP)<br />
may also be introduced into the region to be augmented<br />
and a stable coverage of the defect is ensured.<br />
easy-graft crystal and easy-graft classic are good examples<br />
of successful product maintenance. Its special<br />
handling continues to win over more and more supporters,<br />
enjoying numerous well-documented examples<br />
of long-term success. In contact with blood, the<br />
material will take only a few minutes to solidify into a<br />
porous body that has the same shape as the defect,<br />
which will often render membrane coverage unnecessary.<br />
The difference between easy-graft classic and<br />
easy-graft crystal is in their chemical composition,<br />
making them suitable for different indications. “Classic”<br />
easy-graft consists mainly of ß-TCP. It is fully resorbed<br />
by the body and replaced by bone. By contrast,<br />
easy-graft crystal is only partially resorbed. It consists<br />
of coated biphasic calcium phosphate (40% ß-TCP,<br />
60% HA). The hydroxyapatite fraction remains integrated<br />
in the bone, ensuring sustained volume stability.<br />
Dental Bone Bone &<br />
Tissue Tissue Regeneration<br />
Alpha-Bio A lpha-Bio<br />
Tec, Tec,<br />
lleading<br />
ea<br />
d ding<br />
innovator<br />
in<br />
aadvanced<br />
dvanced<br />
iimplantology<br />
mplantology<br />
solution,<br />
ppresents<br />
resents<br />
AAlpha-Bio's<br />
lpha-Bi<br />
o o's<br />
GRAFT<br />
- the<br />
new<br />
hhigh<br />
igh<br />
qquality<br />
uality<br />
bbone<br />
one<br />
and<br />
tissue<br />
regeneration<br />
pproduct<br />
roduct<br />
lline<br />
ine<br />
tthat<br />
hat<br />
ooffers<br />
offers<br />
safe<br />
and<br />
rreliable<br />
eliable<br />
ssolutions<br />
olutions<br />
ffor<br />
o or<br />
a variety<br />
of<br />
ddental<br />
ental<br />
requirements<br />
and<br />
indications.<br />
SSynthetic,<br />
ynthetic,<br />
RResorbable<br />
R e s o r b a b l e<br />
Bone<br />
Natural<br />
Bovine Bo<br />
v vine<br />
Bone Bo<br />
n ne<br />
Collagen Collag<br />
e en<br />
Membrane<br />
Me<br />
m b brane<br />
New<br />
Simple<br />
Solutions<br />
ffor<br />
or<br />
CComplex<br />
omplex<br />
Cases<br />
Collagen<br />
Fleece<br />
VVisit<br />
isit<br />
uus<br />
s aat<br />
t IIDS<br />
DS<br />
CCologne<br />
olog<br />
n ne<br />
MMarch<br />
arch<br />
222-26,<br />
2-26,<br />
22011<br />
011<br />
Hall<br />
4.2, 4.<br />
2,<br />
Stand<br />
G-020
108 <strong>EDI</strong><br />
Product News<br />
Omnia Circular scalpel<br />
Aseptico Elite<br />
The Elite all-in-one motor system from Aseptico<br />
(model AEU-7000L-70V) is indicated for a number of<br />
dental specialties, including implant, endo, restoration,<br />
and oral surgery. Features include:<br />
• A powerful, 40K rpm, autoclavable micromotor<br />
with LED illumination at any speed. Unlike conventional<br />
fiber optic bulbs, the integrated LED<br />
provides tens of thousands of operating hours.<br />
• An advanced dynamometer calibration system<br />
that automatically detects the properties of individual<br />
implant or endo reduction handpieces for<br />
highly accurate speed, torque, and efficiency<br />
results at the time of treatment. This means that<br />
almost every brand of new or existing reduction<br />
handpiece from 16:1 – 32:1 (implant) and 4:1 – 16:1<br />
(endo) can be used.<br />
• Torque control is adjustable up to 60 Ncm,<br />
ensuring compatibility with every implant system<br />
in the market.<br />
• Endo functionality that exceeds any competing<br />
hybrid system (on-board file library, torque in<br />
g-cm, auto-stop-reverse, etc.).<br />
• Twelve programmable preset buttons<br />
(six implant/six endo) are available for saving<br />
preferred ratio, speed, torque, irrigation, motor<br />
direction settings, and even display names.<br />
• Upgradable software ensures longevity of the<br />
device.<br />
The circular scalpel or soft-tissue<br />
punch is used to precisely incise<br />
the mucosal rim around the<br />
implant. Omnia offers scalpels<br />
with three different diameters<br />
(Ø 4.1, Ø 5.2 and Ø 6.2) for easier<br />
adaptation to major implants<br />
brands.<br />
Since the incision is limited to<br />
the dimensions of the implant<br />
cap, the disposable circular scalpel<br />
causes less mucosal trauma than<br />
traditional scalpels.<br />
The Elite performs the complete<br />
implant procedure using a 20:1<br />
reduction contra angle, such as<br />
the new AHP-85MBFO-CX Mont<br />
Blanc Fiber Optic Handpiece with<br />
Depth Control. The micromotor is<br />
also compatible with most brands<br />
of lighted and non-lighted handpieces<br />
(such as the AHP-85MB-X<br />
or AHP-85MB-CX Mont Blanc and<br />
the AHP-85P-I Impulsion). The<br />
Elite may also be used with 8:1<br />
endo, 1:1 standard, 1:2, and 1:5<br />
increaser handpieces for a multitude<br />
of dental applications.<br />
Product:<br />
Circular scalpel<br />
Indication:<br />
Dental implantology, oral surgery<br />
and maxillofacial surgery<br />
Distribution:<br />
Omnia S.p.A<br />
Via F. Delnevo 190<br />
43036 Fidenza (PR) · Italy<br />
Phone: +39 0524 527453<br />
info@omniaspa.eu<br />
www.omniaspa.eu<br />
Product:<br />
Elite<br />
Indication:<br />
LED motor system<br />
Distribution:<br />
Aseptico<br />
P.O. Box 1548<br />
Woodinville, WA 98072<br />
USA<br />
Phone: +1 425 487-3157<br />
info@aseptico.com<br />
www.aseptico.com
It’s Time for a ReThink:<br />
High Quality at factory-direct Prices<br />
Zimmer © Dental *<br />
Implant: from €100<br />
In times of financial constraints -<br />
look for innovation with the best value!<br />
Implant Direct sets new standards with high-quality implants with low prices<br />
and value added All-in-One Packaging for only 115 Euro per implant, including<br />
the corresponding prosthetic components. Besides the unique Spectra System,<br />
we offer compatible implant systems to Nobel BiocareTM , Str aumann and Zimmer ©<br />
Dental. Make a decision today and choose a path of smart solutions and<br />
considerably more profit.<br />
*Registered trademarks of Straumann, Zimmer © Dental and Nobel Biocare TM<br />
Spectra-System<br />
Six application-specific implants<br />
All-in-One O e Package: ac age € 1155<br />
Hexagon Tri-Lobe Octagon<br />
Nobel Biocare TM *<br />
RePlant Line<br />
RePlus Line<br />
ReActive Line<br />
All-in-One Package: from € 115<br />
All-in-One Package: includes implant, abutment, cover screw, healing collar, comfort cap and transfer<br />
Straumann *<br />
SwishPlant Line<br />
All-in-One Package: € 145<br />
Innovation with the best value<br />
Hall 4.1<br />
Stand E018�F019<br />
and Hall 10.1<br />
Stand J029<br />
Visit us!<br />
Tollfree Infoline: 00800 4030 4030<br />
www.implantdirect.eu<br />
Europe’s No. 1 Online Provider for Dental Implants<br />
�������������������������������������������������� ������������������������
110 <strong>EDI</strong><br />
Product News<br />
Leader Italia Tixos Nano<br />
The Tixos family of implants has received two new<br />
members, based on the familiar and much-appreciated<br />
Nano micro-implants. Leader Italia’s two new<br />
implants, the Tixos Nano 2 and the Tixos Nano OVD,<br />
owe their advantages to further optimization of the<br />
production process and a number of changes to the<br />
implant design – without in any way compromising<br />
the benefits of the high-quality Tixos surface.<br />
The new products differ from Tixos Nano implants<br />
in that their neck is shorter. Moreover, the Normo ball<br />
of the OVD implants is not nitride-treated.<br />
The Tixos Nano 2 and the Nano OVD feature the<br />
new Tixos laser-microfused titanium surface: An<br />
isoelastic surface is created around a very compact<br />
core, replicating the spongy structure of natural bone<br />
by micro- and macro-cavities of well-defined size and<br />
shape interconnected by micro-pores. The structure<br />
is highly mimetic, accelerating bone healing and<br />
osseointegration, as demonstrated by in-vitro and<br />
in-vivo human studies*.<br />
*References available on www.leaderitalia.it.<br />
The new Saturno Pivoting O-Ring Attachment from<br />
Zest Anchors makes restoring overdentures using<br />
O-rings much simpler and more predictable. This<br />
innovative new overdenture attachment features a<br />
race that pivots 15 degrees in any direction inside<br />
the denture cap while securely holding the O-ring.<br />
These attachments compensate for divergent im -<br />
plant angles by creating an aligned fit that is forgiving<br />
and patient friendly. Extensive laboratory<br />
testing has demonstrated that the Saturno O-Ring<br />
Attachment outperforms traditional O-rings during<br />
insertion and removal at 15 degrees of divergence.<br />
Saturno is available for standard and mini implant<br />
applications. A new, patented O-Ring Insertion Tool<br />
that makes replacing O-rings quick and efficient is<br />
also offered by Zest Anchors.<br />
Product:<br />
Tixos Nano<br />
Indication:<br />
Micro-implant with shorter<br />
transmucosal segment<br />
Distribution:<br />
Leader Italia Srl<br />
Via Aquileja 49<br />
20092 Cinisello Balsamo (MI)<br />
Italy<br />
Phone: +39 02 618651<br />
export@leaderitalia.it<br />
www.leaderitalia.it<br />
Zest Anchors<br />
Saturno Pivoting O-Ring Attachment<br />
Product:<br />
Zest Saturno Pivoting O-Ring<br />
Attachment<br />
Indication:<br />
Restoration of overdentures<br />
using O-rings<br />
Distribution:<br />
Zest Anchors, LLC<br />
2061 Wineridge Place<br />
Escondido, CA 92029<br />
USA<br />
Phone: +1 760 743-7744<br />
www.zestanchors.com
Europe<br />
Bulgaria<br />
Croatia<br />
France<br />
Georgia<br />
Germany<br />
Italy<br />
+359 285 910 24<br />
+38 549 372 605<br />
+33 545 839 452<br />
+99 532 9100 00<br />
+49 6196 777 550<br />
+39 185 788 7863<br />
Lithuania<br />
Norway<br />
Poland<br />
Portugal<br />
Russia<br />
Serbia<br />
+370 521 530 62<br />
+47 229 991 41<br />
+48 22 6581444<br />
+351 968 205 696<br />
+7 496 739 9925<br />
+381 112 763 639<br />
Slovakia<br />
Spain<br />
Sweden<br />
Turkey<br />
United Kingdom<br />
+421 527 722 924<br />
+34 934 239 294<br />
+46 8 732 6170<br />
+90 216 418 5100<br />
+44 773 915 6413<br />
Africa<br />
OSSTEM Germany GmbH Mergenthalerallee 25, 65760 Eschborn, Germany<br />
europe@osstem.com www.sinuskit.com<br />
Middle East<br />
Iran +98 218 861 0256<br />
Pakistan +92 321 2422365<br />
Saudi Arabia +966 128 130 08<br />
Syria +963 114 416 714<br />
Egypt +202 3304 3554
112 <strong>EDI</strong><br />
112<br />
Product News<br />
Leone Exacone implant system<br />
Patient’s restorative needs are increasing daily. Dental<br />
professionals are called upon to meet these needs.<br />
With new digital technologies making ever stronger<br />
inroads on the world of dentistry, the products used in<br />
these technologies have gone from “nice to have” to<br />
“impossible to do without”.<br />
The Exacone implant with its screwless Morse taper<br />
connection simplifies and improves the application of<br />
most recent technologies. With this implant, high-resolution<br />
digital impressions no longer have to depend<br />
on special accessories such as scanning abutments,<br />
but can be taken directly on the abutments themselves.<br />
Regardless of whether an intraoral scanner or a<br />
laboratory scanner is used.<br />
The system’s prosthetic advantages are further enhanced<br />
by CAD/CAM techniques, as the absence of the<br />
screw access channel greatly simplifies the design and<br />
fabrication of frameworks. In particular the MultiTech<br />
abutments have been designed to more readily respond<br />
to the requirements of CAD/CAM technology,<br />
which favours their use in custom solutions.<br />
Aesculap will be presenting its new instruments for<br />
the tunnelling technique at this year’s IDS in Cologne.<br />
The tunnelling instruments are characterized by discshaped<br />
working ends offering considerable extra freedom<br />
of movement in frontal and lateral subperiosteal<br />
preparations using the tunnelling technique in connective-tissue<br />
graft procedures.<br />
Strategically sharpened at the edges, these instruments<br />
facilitate not only the tunnelling technique<br />
but also sulcular preparation as used in the envelope<br />
technique. The design and curvature of the working<br />
Exacone implants are included in<br />
the libraries of most common implant<br />
treatment planning systems,<br />
facilitating computer-assisted management<br />
of implant therapy at<br />
every step from implant placement<br />
to prosthetic restorations.<br />
The Exacone system is perfectly in<br />
tune with today’s high-tech equipment,<br />
ready to contribute its share<br />
to dentistry’s digital revolution.<br />
ends greatly simplify preparation<br />
tasks even on convex surfaces. In<br />
this manner, application of the<br />
tunnelling technique is simplified<br />
for users, allowing them to<br />
achieve significantly improved results.<br />
Aesculap’s new tunnelling instruments<br />
will be available in a<br />
straight (DX310R) and an angled<br />
(DX311R) version.<br />
Product:<br />
Exacone implant system<br />
Indication:<br />
Dental implantology<br />
Distribution:<br />
Leone S.p.A.<br />
Via P. a Quaracchi, 50<br />
50019 Sesto Fiorentino (Fi)<br />
Italy<br />
Phone: +39 055 3044-1<br />
info@leone.it<br />
www.leone.it<br />
Aesculap New tunnelling instruments<br />
Products:<br />
Tunnelling instruments<br />
Indication:<br />
Dental periodontology and<br />
implantology<br />
Distribution:<br />
Aesculap AG<br />
Am Aesculap-Platz<br />
78532 Tuttlingen<br />
Germany<br />
Phone: +49 7461 95-0<br />
dental@aesculap.de<br />
www.aesculap-dental.com
· Universal connection<br />
· Anatomic design<br />
· Compatible<br />
High Definition<br />
Every single feature defines a Masterpiece<br />
Are you interested in becoming our distributor?<br />
Contact us.<br />
HEADQUARTERS<br />
· Non-traumatic apex<br />
· RBM Surface treatment<br />
· Triple-thread Design<br />
Visit us at IDS 2011<br />
34 th International Dental Show<br />
Cologne, 22-26.3.2011<br />
Stand D78. Hall 4.1<br />
· 11º Morse taper connection<br />
· Platform switching<br />
· Microthreads<br />
· 45º implant shoulder<br />
· Immediate loading<br />
· RBM Surface treatment<br />
C/ Santiago López González, 7 · 47197 Valladolid · Spain · Tel. +34 983 211 312<br />
Fax +34 983 304 021 · info@mozo-grau.com · www.mozo-grau.com<br />
CHILE CHINA COLOMBIA POLAND PORTUGAL SPAIN TAIWAN VENEZUELA
114 <strong>EDI</strong><br />
Product News<br />
Dentaurum Implants tioLogic pOsition<br />
Modern 3D imaging technologies such as DVT and<br />
CT give users a better view of existing jaw structures<br />
and bone conditions before surgical procedures,<br />
allowing them to determine the implant positions<br />
and to select the right protocols.<br />
The tioLogic pOsition navigation system has been<br />
designed to work with imaging processes and 3D<br />
planning software for guided preparation, followed by<br />
placement of tioLogic implants. If the indication al-<br />
lows it, tioLogic implants can be restored<br />
immediately with previously<br />
fabricated restorations.<br />
The tioLogic pOsition system includes<br />
specially designed instruments<br />
and accessory components<br />
for preparing the bone site and for<br />
implant placement. The sleeves of<br />
the tioLogic pOsition system are<br />
made of titanium and ensure accurate<br />
guidance of the drills. The bone<br />
is atraumatically prepared to the<br />
specified implant length while<br />
gradually widening the diameter.<br />
Working with the tioLogic pOsition<br />
has been designed to be simple<br />
and safe for users with the inclusion<br />
of many special details<br />
such as the ability to adjust the<br />
handle of the internal sleeves in<br />
three dimensions for work in restricted<br />
spaces or the silicone ring<br />
that fixes the internal sleeves in<br />
position during the procedure.<br />
Alpha-Bio Tec Alpha Universe<br />
MultiUnit abutment system<br />
Alpha-Bio Tec has launched the Alpha Universe Multi-<br />
Unit angular abutment system for use with tilted<br />
implants. It is simple and easy to use and consists<br />
of two parts: The base (UniBase), available in a range<br />
of angles and heights, and the cover (UniCover), available<br />
in a range of designs according to the desired<br />
restoration option. The MultiUnit body is supplied<br />
connected to a patent pending, flexible plastic handle,<br />
which enables precise base placement, even in<br />
hard-to-reach places. The specially designed base and<br />
cover are screwed together in order to provide extra<br />
strength and stability, assuring a long-term solution.<br />
Alpha Universe is compatible with the standard<br />
Alpha-Bio Tec tools and prosthetic parts to reduce<br />
inventory and to simplify the restoration process.<br />
The UniBase is available in 17° and 30° angles, and 1.5<br />
and 2.5 mm heights. The UniBase kit contains the base,<br />
connected to the flexible handle and two UniScrews –<br />
a blue screw for the physician and<br />
a silver screw for the lab.<br />
The range of UniCovers enables<br />
a wide range of restoration options<br />
– screw retained, bar retained,<br />
ball retained and cement<br />
retained. UniCovers are also available<br />
in a range of heights for optimal<br />
aesthetics.<br />
Product:<br />
tioLogic pOsition<br />
Indication:<br />
Guided implant placement<br />
Distribution:<br />
Dentaurum Implants GmbH<br />
Turnstr. 31<br />
75228 Ispringen<br />
Germany<br />
Phone: +49 7231 803-560<br />
info@dentaurum-implants.de<br />
www.dentaurum-implants.de<br />
Product:<br />
Alpha Universe MultiUnit<br />
abutment system<br />
Indication:<br />
Angular abutment system<br />
Distribution:<br />
Alpha-Bio Tec Ltd.<br />
7 Hatnufa St. · P.O.B 3936<br />
Petach Tikva 49510<br />
Israel<br />
Phone: +972 3 9291055<br />
info@alpha-bio.net<br />
www.alpha-bio.net
Malta 2011<br />
International Anniversary Congress<br />
Innovation through cooperation – your partner on the way to success<br />
September 22 - 25, 2011<br />
Register now !<br />
Dentaurum Implants GmbH<br />
· Dentauru m Im plants G m bH ·<br />
Prof. Dr. Ahmed Barakat · University Cairo · Egypt n Prof. Dr. Tobias M. Böckers · University Ulm · Germany<br />
Prof. Dr. Christoph Bourauel · University Bonn · Germany n Prof. Dr. Marzena Dominiak · University Wroclaw · Poland<br />
Prof. Dr. Dr. Wilfried Engelke · University Göttingen · Germany n Dr. James Galea · Malta<br />
Prof. Dr. Tomas Gedrange · University Greifswald · Germany n Dr. Friedhelm Heinemann · Germany<br />
Dr. Joachim Hoffmann · Germany n Dr. Peter Keller · Germany n Dr. Alireza Keshvad · Iran<br />
ZT Björn Koller · Germany n Dr. Stephan Kressin · Germany n Dr. Friedemann Petschelt · Germany<br />
Dr. Umberto Pratella · Italy n Dr. Hatem W. Al Rashdan · Jordan n ZT Germano Rossi · Italy<br />
Prof. Dr. Klaus Roth · University Hamburg · Germany n Dr. Enzo de Santis · Italy n Dr. Daniel Schulz · Germany<br />
Dr. Sigmar Schnutenhaus · Germany n Dr. Manfred Sontheimer · Germany<br />
Turnstraße 31 · 75228 Ispringen · Germany · Phone + 49 72 31 / 803 - 0 · Fax + 49 72 31 / 803 - 295<br />
www.dentaurum-implants.de · E-Mail: info@dentaurum-implants.de<br />
DE_0211_Anzeige_Malta_Implants_EN_210x297mm.indd 1 03.02.2011 15:10:58 Uhr
116 <strong>EDI</strong><br />
Product News<br />
Mozo-Grau<br />
17° Angled Tapered Multi-Unit Abutment<br />
Mozo-Grau, manufacturer of the MG InHex and<br />
MG Osseous dental implant systems, now offers<br />
an option for screw-retained restorations on angled<br />
tapered abutments developed by Mozo-Grau’s engineering<br />
department.<br />
This new abutment has been designed for screwretained<br />
restorations with a screw angle of 17° in relation<br />
to the implant insertion axis. This feature is very<br />
useful in difficult situations or in multi-implant protocol<br />
cases, such as the “All-on-4” technique that entails<br />
angled insertion on the implants placed at the distalmost<br />
positions.<br />
The multi-unit solution will shortly be complemented<br />
by abutments with other angulations.<br />
Implant Direct Sybron has added two very interesting<br />
lines of regenerative products to its product offering<br />
– Bioresorb Macro Pore grafting material and<br />
Cytoplast membranes and sutures.<br />
Bioresorb Macro Pore is an osteoconductive, purephase<br />
ß-tricalcium phosphate (99%) bone-grafting<br />
material developed via a patented process that creates<br />
a highly porous structure very similar to human<br />
bone. The combination of interconnecting micro- and<br />
macroporous structures gives this material its definitive<br />
advantage by allowing vascularization and the<br />
introduction of cells into and through the particle. This<br />
in turn results in new bone deposits within the parti-<br />
cles themselves rather than just on<br />
the particle surface, as can be the<br />
case with less porous materials.<br />
The Cytoplast line of membranes<br />
gives clinicians the choice<br />
between a resorbable membrane<br />
made of highly purified type 1 collagen<br />
or a non-resorbable membrane<br />
made of d-PTFE if primary<br />
closure of the grafting site cannot<br />
be achieved. For cases with a defect<br />
affecting one or more walls or<br />
where head space at the grafting<br />
site needs to be maintained, premium<br />
non-resorbable membranes<br />
with titanium reinforcements are<br />
available in various shapes and<br />
sizes.<br />
Both products are indicated for<br />
treating most osseous defects and<br />
result in highly predictable bone<br />
formation. You can learn more<br />
about the products at IDS 2011.<br />
Visit the two Implant Direct Sy -<br />
bron booths in Hall 4.1, Stand F18/<br />
E19 and Hall 10.1, Stand J29.<br />
Product:<br />
17° Angled Tapered Multi-Unit<br />
Abutment<br />
Indication:<br />
Free-standing implants,<br />
overdenture treatment<br />
Distribution:<br />
Mozo-Grau<br />
Santiago González López 7<br />
47197 Valladolid<br />
Spain<br />
Phone: +34 983 211-312<br />
sales@mozo-grau.com<br />
www.mozo-grau.com<br />
Implant Direct Sybron Extended product range<br />
Products:<br />
Bioresorb Macro Pore grafting<br />
material; Cytoplast membranes<br />
and sutures<br />
Indication:<br />
Treatment of osseous defects<br />
Distribution:<br />
Implant Direct Europe AG<br />
Hardturmstrasse 161<br />
8005 Zürich<br />
Switzerland<br />
Phone: 00800 40304030<br />
info@implantdirect.eu<br />
www.implantdirect.eu
Anteriores –<br />
Natural and Beautiful Teeth<br />
by Jan Hajto<br />
Picture Gallery<br />
This book aims to be highly visual and inspiring. A selection of naturally beautiful anterior teeth<br />
is presented in the form of a coloured atlas. The cases are systematically arranged according to<br />
gender and an approximate classifi cation of the regularity of the dentition.<br />
This collection will become indispensable as your manual for the aesthetic planning and production<br />
of anterior restorations or as an aid to communication between dentist, patient and dental<br />
technician.<br />
Implant Retained Single Tooth Restorations<br />
by Roberto Bellini<br />
The production of single tooth crowns in the<br />
anterior tooth or lateral tooth area has always<br />
posed a special challenge to the technical and<br />
creative competence of a dental technician. In<br />
this reference book are more brilliant pictures<br />
than explanatory text because the author<br />
consciously tried to use the medium ‘picture’<br />
as a prime information transfer.<br />
103 pages, 254 pictures<br />
ISBN: 978-3-932599-09-5<br />
REF 9009 € 24,80<br />
270 pages, 950 illustrations<br />
ISBN: 978-3-932599-19-4<br />
Crown – Bridge & Implants<br />
by Luc & Patrick Rutten<br />
REF 9019 € 153,-<br />
The authors show in this book the way to a<br />
perfect red and white aesthetic by using about<br />
1,300 excellent pictures and a cross section<br />
out of their daily work in the laboratory. An<br />
essential schoolbook for the prosthetic and<br />
implant logical dentist and dental technician.<br />
296 pages, 1300 pictures<br />
ISBN: 978-3-932599-17-0<br />
REF 9021 € 149,-
118 <strong>EDI</strong><br />
Product News<br />
Nobel Biocare Replace Select TC<br />
Replace Select TC is a two-piece implant with a 3 mm<br />
high, extended machined collar, which enables platform<br />
access at tissue level. Simultaneously, its colorcoded<br />
internal tri-channel connection facilitates<br />
accurate and quick restorative component identification<br />
and placement.<br />
The body of Replace Select TC is derived from Brånemark<br />
System MK III implants, making it well-suited<br />
for all bone types and one-stage treatment protocols.<br />
Zimmer Dental Inc. is studying ways in which Trabecular<br />
Metal Technology can be applied to the dental<br />
market. Manufactured at Zimmer’s TMT facility in<br />
Parsippany, NJ, USA, Trabecular Metal Material has<br />
been used in the company’s cutting-edge ortho pae -<br />
dic devices for more than a decade.<br />
Trabecular Metal Material will be presented at IDS<br />
2011. It is a three-dimensional, highly biocompatible<br />
material – not an implant surface or coating – with a<br />
structure, function, and flexibility comparable to cancellous<br />
bone. Made from tantalum, element number 73<br />
in the periodic table, Trabecular Metal Material is fabricated<br />
utilizing a proprietary vapor deposition process.<br />
A glimpse inside Trabecular Metal Material reveals<br />
its uniform, three-dimensional cellular architecture<br />
with up to 80 percent porosity and a surface area<br />
exhibiting a consistent, nanotextured topography.<br />
While conventional textured or coated implant surfaces<br />
achieve bone-to-implant contact, or on-growth,<br />
Trabecular Metal Material’s open and interconnected<br />
network of pores is designed for both on-growth<br />
and in-growth, or osseoincorporation. This means<br />
that bone has the potential to not only grow into the<br />
pores and around the struts, but also to interconnect.<br />
In a retrospective study with an<br />
average three year follow-up, Replace<br />
implants with 3 mm collars<br />
demonstrated a cumulative survival<br />
rate of 99.2 per cent.<br />
To facilitate immediate temporization<br />
using existing dentures<br />
and to prevent soft tissue overgrowth,<br />
a new healing screw was<br />
developed for Replace Select TC.<br />
Two different screws are available<br />
to accommodate soft tissue thickness<br />
and facilitate intraoperative<br />
flexibility: 1 mm and 3 mm height<br />
variants. Final prosthetics can be<br />
attached to Replace Select TC using<br />
any Nobel Biocare overdenture<br />
solution: Locator Abutment,<br />
Ball Abutment, Gold Abutment Bar/<br />
Gold Coping Bar and NobelProcera<br />
Implant Bar Overdenture.<br />
While other manufacturers<br />
have attempted to mimic the<br />
properties of Trabecular Metal<br />
Technology through sintered<br />
bead and other conventional<br />
porous coatings and materials,<br />
the end products have differed<br />
significantly. The cancellous-like<br />
structure, interconnected porosity<br />
and in-growth potential are a<br />
special combination of attributes<br />
that contribute to the osteoconductive<br />
properties of Trabecular<br />
Metal Material.<br />
Product:<br />
Replace Select TC<br />
Indication:<br />
Two-piece dental implant<br />
Distribution:<br />
Nobel Biocare Management AG<br />
P.O. Box<br />
8058 Zurich-Airport<br />
Switzerland<br />
Phone: +41 43 2114200<br />
info@nobelbiocare.com<br />
www.nobelbiocare.com<br />
Zimmer Dental Trabecular Metal Material<br />
Product:<br />
Trabecular Metal Material<br />
Indication:<br />
Dental implantology<br />
Distribution:<br />
Zimmer Dental Inc.<br />
USA<br />
Phone Germany: +49 761 15647-0<br />
Phone Spain: +34 93 84605-43<br />
Phone France: +33 1 451235-66<br />
Phone Italy: +39 043 85555-73<br />
Phone Israel: +972 3 612-4242<br />
www.zimmerdental.com
<strong>EDI</strong> 119<br />
Product News
120 <strong>EDI</strong><br />
Product News<br />
mectron Bone expanders<br />
At IDS 2011, mectron will be presenting its bone<br />
expanders, indicated mainly for splitting the atrophic<br />
alveolar ridge and for the lateral bone-condensation<br />
technique. These bone expanders were developed<br />
in scientific collaboration with Dr Rosario<br />
Sentineri. They are available in three different diame -<br />
ters (2.5 mm/3.5 mm/4.5 mm) and two lengths<br />
(11.5 mm/15 mm) to suit different anatomical situations.<br />
All of them can be used with either an implantology<br />
micromotor or a manual ratchet with dedicated<br />
adapters.<br />
mectron will be showing various exciting new<br />
products at its IDS Cologne booth (Hall 10.2, Stand<br />
The Locator Attachment for overdentures from Zest<br />
Anchors is designed with the primary benefits of<br />
ease of insertion and removal, customizable levels of<br />
retention, low vertical profile and durability. The most<br />
critical design feature is its innovative ability to pivot,<br />
which increases the attachment’s resiliency and tolerance<br />
for the high mastication forces an attachment<br />
must withstand. This patented pivoting feature also<br />
allows the attachment to compensate for implant<br />
divergence. Today, Locator has become the overdenture<br />
attachment that is embraced by clinicians<br />
worldwide. It is currently available for over 350 different<br />
implants produced by more than 70 manufactur-<br />
Kohler Paralleling guide<br />
O40/P41). Visitors can enjoy the<br />
new products while sipping a free<br />
tasty espresso or two.<br />
ers, meaning almost any implant<br />
platform has a compatible Locator<br />
Attachment to fit.<br />
The paralleling guide is used for<br />
aligning two or four interforaminal<br />
implants in the edentulous<br />
mandible with the median plane. A<br />
hole is drilled in the middle of the<br />
mandible using a pilot drill, which<br />
is easily aligned with the median<br />
plane. The distance from the middle<br />
can be accurately transferred<br />
to the opposite side by swivelling<br />
the fitted paralleling guide.<br />
Products:<br />
Bone expanders<br />
Indication:<br />
Dental implantology<br />
Distribution:<br />
mectron s.p.a.<br />
via Loreto 15A<br />
16042 Carasco (GE) · Italy<br />
Phone: +39 0185 35361<br />
mectron@mectron.com<br />
www.mectron.com<br />
Zest Anchors Locator Implant Attachment<br />
Product:<br />
Zest Locator Implant Attachment<br />
Indication:<br />
Overdenture attachment<br />
Distribution:<br />
Zest Anchors, LLC<br />
2061 Wineridge Place<br />
Escondido, CA 92029<br />
USA<br />
Phone: +1 760 743-7744<br />
www.zestanchors.com<br />
Product:<br />
Paralleling guide according to<br />
Dr Schäfer, Germany<br />
Indication:<br />
Dental implantology<br />
Distribution:<br />
Kohler Medizintechnik GmbH & Co. KG<br />
Bodenseeallee 14-16<br />
78333 Stockach · Germany<br />
Phone: +49 7771 64999-0<br />
info@kohler-medizintechnik.de<br />
www.kohler-medizintechnik.de
Hi Tec Implants<br />
Logic plus implant system<br />
Product:<br />
Logic plus implant system<br />
Indication:<br />
Dental implantology<br />
Distribution:<br />
Hi Tec Implants<br />
P.O. Box 2022<br />
Herzliya<br />
Israel<br />
Phone: +972 9 9587775<br />
sales@hitec-implants.com<br />
www.hitec-implants.com<br />
Trinon GIP implant<br />
Product:<br />
GIP implant<br />
Indication:<br />
Ultra-short<br />
hollow-cylinder implant<br />
Distribution:<br />
Trinon Titanium GmbH<br />
Augartenstraße 1<br />
76137 Karlsruhe<br />
Germany<br />
Phone: +49 721 932700<br />
trinon@trinon.com<br />
www.trinon.com<br />
The Logic plus implant system by<br />
Hi Tec Implants was developed<br />
with the vision of providing the<br />
ultimate range of products for all<br />
needs and requirements, covering<br />
all clinical indications – for maximum<br />
simplicity, using only one<br />
prosthetic system for all implant<br />
diameters. The implant system<br />
features platform switching to preserve<br />
crestal bone. The prosthetic<br />
system is extensive and covers:<br />
• A universal abutment system<br />
for screw-retained bridges,<br />
including angulated universal<br />
abutments for posterior cantilever<br />
situations and full-arch<br />
rehabilitations.<br />
• A modular abutment system,<br />
snap-ons, zirconia and castgold<br />
abutments and a large<br />
selection of titanium abutments.<br />
The GIP implant by Trinon is an<br />
ultra-short hollow-cylinder im -<br />
plant 7 mm in diameter and 4 to<br />
7 mm in length. Like other Q-Im -<br />
plants by Trinon, the GIP implant<br />
features the Osteo thread and<br />
SLA surface treatment both on<br />
the inner and on the outer surfaces.<br />
Its hollow-cylinder shape<br />
greatly increases the available<br />
<strong>EDI</strong> 121<br />
Product News<br />
The Logic plus system includes the X-6 implant system,<br />
extra short implants with diameters of 4.30 mm, 5.0 mm<br />
and 6.0 mm and a special thread design for cases of<br />
extremely minimal bone height.<br />
contact surface for osseointegration – a GIP implant<br />
5 mm in length has the same surface area as a<br />
tapered Q-Implant 4.5 mm in diameter and 12 mm in<br />
length.<br />
Patients with a preference for a fixed rehabilitation<br />
supported by implants often cannot receive<br />
their treatment of choice due to an insufficient<br />
bone supply. Some of these patients have benefitted<br />
from the advent of angulated implants. With ultrashort<br />
implants now available, dentists and patients<br />
now have additional treatment options at their disposal<br />
– the GIP implant has a diameter of 7 mm<br />
and an inside diameter of 5 mm is an excellent<br />
choice. Rotational stability of the implant is ensured<br />
by the stable bone connection afforded by the internal<br />
cylinder and the four longitudinal cuts 2 mm<br />
below the implant shoulder. A platform-switching<br />
effect is established by the micro-groove design of<br />
the wide shoulder. Clinical experiences over the past<br />
five years have proven ultra-short implants to be a<br />
safe alternative to bone augmentation.
122 <strong>EDI</strong><br />
Calendar of Events<br />
CALENDAR OF EVENTS<br />
2011 Event Location Date Details/Registration<br />
March 34th International Dental Show<br />
(IDS) 2011<br />
Cologne, Germany 22–26 March 2011 Koelnmesse GmbH<br />
Phone: +49 180 577-3577<br />
www.ids-cologne.de<br />
April Forum Dental Mediterraneo Barcelona, Spain 7–9 April 2011 Puntex<br />
Phone: +34 937 964-507<br />
www.puntex.es/fdm/index.php<br />
Scandefa 2011 Copenhagen, Denmark 7–9 April 2011 Bella Center<br />
www.scandefa.dk<br />
International Osteology<br />
Symposium 2011<br />
May 360° Implantology – MIS Global<br />
Conference 2011<br />
June 5th BDIZ <strong>EDI</strong> Mediterranean<br />
Symposium<br />
July Advanced Certificate Course in Sinus<br />
Grafting and Bone Augmentation<br />
with Hands-on Cadaver Surgery<br />
September FDI Annual World Dental Congress<br />
2011<br />
Joint congress of BDIZ <strong>EDI</strong> and<br />
DGOI<br />
October EAO Annual Scientific Congress<br />
2011<br />
<strong>EDI</strong> – Information for authors<br />
<strong>EDI</strong> – the interdisciplinary journal for prosthetic dental implantology is<br />
aimed at dentists (and technicians) interested in prosthetics implantology.<br />
All contributions submitted should be focused on this aspect in content<br />
and form. Suggested contributions may include:<br />
. Case studies<br />
. Original scientific research<br />
. Overviews<br />
Manuscript submission<br />
Submissions should include the following:<br />
. two hard copies of the manuscript<br />
. a disk copy of the manuscript<br />
. a complete set of illustrations<br />
Original articles will be considered for publication only on the condition<br />
that they have not been published elsewhere in part or in whole and are<br />
not simultaneously under consideration elsewhere.<br />
Manuscripts<br />
Pages should be numbered consecutively, starting with the cover page.<br />
The cover page should include the title of the manuscript and the name<br />
and degree for all authors. Also included should be the full postal address,<br />
telephone number, fax number, and electronic mail address of the contact<br />
author. The second page should contain an abstract that summarizes the<br />
article in approximately 100 words.<br />
Manuscripts can be organized in a manner that best fits the specific goals<br />
of the article, but should always include an introductory section, the body<br />
of the article and a conclusion.<br />
Figures and tables<br />
Each article should contain a minimum of 20 and a maximum of 50 origi -<br />
nal color slides (35 mm) or digital photos, except in unusual circumstances.<br />
The slides will be returned to the author after publication. Slides<br />
should be numbered on the mount in the sequential numerical order in<br />
which they appear in the text (Fig. 1, Fig. 2, etc.).<br />
Editors office: teamwork media GmbH, Hauptstr. 1, 86925 Fuchstal/Germany<br />
Phone: +49 8243 9692-0, Fax: +49 8243 9692-22, service@teamwork-media.de<br />
Cannes, France 14–16 April 2011 Osteology Foundation<br />
Phone: +377 97 973555<br />
www.osteology-cannes.org<br />
Cancun, Mexico 19–21 May 2011 MIS<br />
service@mis-implants.com<br />
www.mis-implants.com<br />
Cascais, Portugal 10–17 June 2011 BDIZ <strong>EDI</strong><br />
office@bdizedi.org<br />
www.bdizedi.org<br />
London, England 7–9 July 2011 Dr Koray Feran<br />
Phone: +44 207 224-1488<br />
anai@korayferan.co.uk<br />
Mexico City, Mexico 14–17 September 2011 FDI World Dental Federation<br />
info@fdiworldental.org<br />
www.fdiworldental.org<br />
Munich, Germany 16–17 September 2011 BDIZ <strong>EDI</strong><br />
office@bdizedi.org<br />
www.bdizedi.org<br />
Athens, Greece 13–15 October 2011 Colloquium<br />
Phone: +33 1 4464-1515<br />
www.eao.org<br />
Radiographs, charts, graphs, and drawn figures are also accepted.<br />
Figure legends should be brief one or two-line descriptions of each figure,<br />
typed on a separate sheet following the references. Legends should be<br />
numbered in the same numerical order as the figures.<br />
Tables should be typed on separate sheets and numbered consecutively,<br />
according to citation in the text. The title of the table and its caption<br />
should be on the same sheet as the table itself.<br />
References<br />
Each article should contain a minimum of ten and a maximum of 30 references,<br />
except in unusual circumstances. Citations in the body of the text<br />
should be made in numerical order. The reference list should be typed on<br />
a separate sheet and should provide complete bibliographical information<br />
in the format exemplified below:<br />
[1] Albrektsson, T.: A multicenter report on osseointegrated oral implants.<br />
J Prosthet Dent 1988; 60, 75-82.<br />
[2] Hildebrand, H. F., Veron, Chr., Martin, P.: Nickel, chromium, cobalt dental<br />
alloys and allergic reactions: an overview. Biomaterials 10, 545-548, (1989).<br />
[3] Johanson, B., Lucas, L., Lemons, J.: Corrosion of copper, nickel and gold<br />
dental alloys: an in vitro and in vivo study. J Biomed Mater Res 23, 349,<br />
(1989).<br />
Review process<br />
Manuscripts will be reviewed by three members of the editorial board.<br />
Authors are not informed of the identity of the reviewers and reviewers<br />
are not provided with the identity of the author. The review cycle will be<br />
completed within 60 days. Publication is expected within nine months.<br />
Page charges and reprints<br />
There are no page charges. The publisher will cover all costs of production<br />
and provide the primary author with five free copies of the journal issue<br />
in which the article appears.
YOU’RE THE ONE<br />
I’M AFTER<br />
Innovative German high performance implant system seeks discerning user for<br />
successful permanent relationship. My ideal partner values an exceptionally<br />
strong prosthesis interface, excellent osseointegration, top German quality<br />
at an affordable price, and a wide range of prosthetic components for all<br />
conventional indications. If you too are seeking to forge links on a foundation<br />
of titanium prosthetic components, BEGO cobalt-chrome alloy Wirobond ® MI<br />
and BeCe zirconium for example, drop me a line. I look forward to getting to<br />
know you.<br />
Curious? Meet me at bego-implantology.com<br />
Should you wish, I can also visit you.
XiVE ®<br />
:<br />
Implantology Unlimited<br />
Superior surgical and prosthetic versatility<br />
Outstanding primary stability<br />
even in soft bone<br />
Successful in even the narrowest gaps<br />
with XiVE ®<br />
3.0<br />
Immediate implant restoration<br />
due to the integrated TempBase concept<br />
Greatest possible prosthetic variety<br />
valued worldwide<br />
Discover your implantological freedom with XiVE ®<br />
.<br />
http://xive.dentsply-friadent.com<br />
10 years XiVE ®<br />
Get the Xperience: Lectures,<br />
hands-on, news – hall 11.2<br />
Implantology Unlimited