pdf - 14652 kB - CARNet
pdf - 14652 kB - CARNet
pdf - 14652 kB - CARNet
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
K. JELŠOVSKÁ et al.: NUCLEAR MAGNETIC RESONANCE SPECTRAL FUNCTION AND MOMENTS FOR ...<br />
T may be linearized and the parameters A and θ can be<br />
easily determined. According to equation (5) the following<br />
relation holds:<br />
[ ∆ M ] 2 =<br />
294<br />
1<br />
− T −<br />
2<br />
θ<br />
2 2 ( r − r )<br />
A B B<br />
2 1<br />
.<br />
(14)<br />
The dependence of [ ] 1<br />
M 2<br />
2<br />
−<br />
∆ on temperature is also<br />
shown in Figure 1.<br />
As expected, this dependence is linear and the straight<br />
line drawn through the experimental points is expressed<br />
by equation (14) with the following values of Curie-Weiss<br />
parameters θ and A for NiSO ·1H O: θ = –13 K and A =<br />
4 2<br />
2,026×10 –2K2 . For MnSO ·1H O parameters determined<br />
4 2<br />
by the least squares are: parameter θ = –9,9 K and A =<br />
10,54×10 –2K2 . As the parameters θ and A are known, the<br />
second moment M may be evaluated by means of equation<br />
20<br />
(5). It was done by extraction of the term ( ) 2<br />
2<br />
A⋅ Br T − θ from<br />
the experimental values of M at each measured temperature.<br />
2<br />
By means of this procedure we have obtained the value<br />
of M = (19,50 ± 0,95)×10 20 –8T2 for NiSO ·1H O and for<br />
4 2<br />
MnSO ·1H O is M = (21,30 ± 1,2)×10 4 2 20 –8T 2 .<br />
A negative value of the temperature parameter θ shows<br />
that NiSO ·1H O and MnSO ·1H O should be antiferro-<br />
4 2 4 2<br />
magnetic at the temperatures T < θ [9]. Measurements<br />
of the temperature dependences of the molar magnetic<br />
susceptibility in the temperature range from 5 K up to 300<br />
K (Charles University Prague, Dept. of Metal Physics)<br />
confirmed this conclusion.<br />
By means of NMR measurements<br />
(according to equation<br />
(6)) it is possible to determine<br />
the magnitude of the<br />
magnetic moment µ of para-<br />
i<br />
magnetic ions in a given substance,<br />
however, the structural<br />
parameters A and B have to<br />
i i<br />
be known. We have calculated<br />
them in the approximation<br />
in which the nearest enviroment<br />
of the crystalline water<br />
molecule is formed by two<br />
Me2+ (Me = Mn, Ni) ions. This<br />
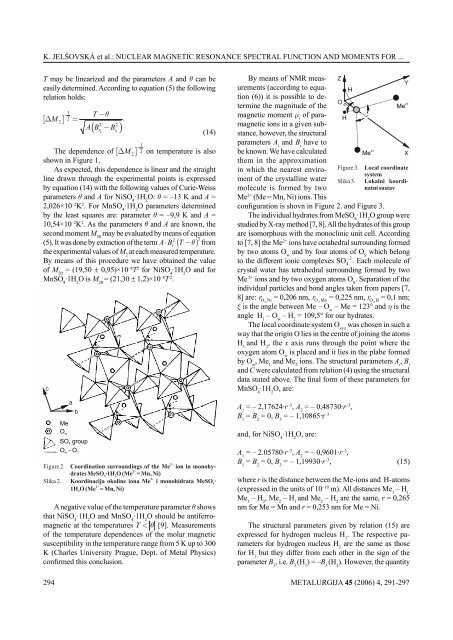
configuration is shovn in Figure 2. and Figure 3.<br />
The individual hydrates from MeSO ·1H O group were<br />
4 2<br />
studied by X-ray method [7, 8]. All the hydrates of this group<br />
are isomorphous with the monoclinic unit cell. According<br />
to [7, 8] the Me2+ ions have octahedral surrounding formed<br />
by two atoms O and by four atoms of O which belong<br />
w S<br />
− 2<br />
to the different ionic complexes SO 4 . Each molecule of<br />
crystal water has tetrahedral surrounding formed by two<br />
Me 2+ ions and by two oxygen atoms O . Separation of the<br />
S<br />
individual particles and bond angles taken from papers [7,<br />
8] are: r Ow Ni = 0,206 nm, r OwMn = 0,225 nm, r OwH = 0,1 nm;<br />
ξ is the angle between Me – O – Me = 123° and η is the<br />
w<br />
angle H – O – H = 109,5° for our hydrates.<br />
1 w 2<br />
The local coordinate system O was chosen in such a<br />
xyz<br />
way that the origin O lies in the centre of joining the atoms<br />
H and H , the x axis runs through the point where the<br />
1 2<br />
oxygen atom O is placed and it lies in the plabe formed<br />
w<br />
by O , Me and Me ions. The structural parameters A , B w 1 2 i i<br />
and C were calculated from relation (4) using the structural<br />
data stated above. The final form of these parameters for<br />
MnSO ·1H O, are:<br />
4 2<br />
A 1 = – 2,17624·r –3 , A 2 = – 0,48730·r –3 ,<br />
B 1 = B 2 = 0, B 3 = – 1,10865·r –3<br />
and, for NiSO 4 ·1H 2 O, are:<br />
A 1 = – 2.05780·r –3 , A 2 = – 0,9601·r –3 ,<br />
B 1 = B 2 = 0, B 3 = – 1,19930·r –3 , (15)<br />
where r is the distance between the Me-ions and H-atoms<br />
(expressed in the units of 10 –10 m). All distances Me 1 – H 1,<br />
Me 1 – H 2 , Me 2 – H 1 and Me 2 – H 2 are the same, r = 0,265<br />
nm for Me = Mn and r = 0,253 nm for Me = Ni.<br />
The structural parameters given by relation (15) are<br />
expressed for hydrogen nucleus H 1 . The respective parameters<br />
for hydrogen nucleus H 2 are the same as those<br />
for H 1 but they differ from each other in the sign of the<br />
parameter B , i.e. B (H1 ) = –B (H2 ). However, the quantity<br />
3 3<br />
3<br />
METALURGIJA 45 (2006) 4, 291-297