A GUIDE TO CAROTENOID ANALYSIS IN FOODS
A GUIDE TO CAROTENOID ANALYSIS IN FOODS
A GUIDE TO CAROTENOID ANALYSIS IN FOODS
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
14 A Guide to Carotenoid Analysis in Foods<br />
SOME PHYSICOCHEMICAL PROPERTIES OF <strong>CAROTENOID</strong>S<br />
A good understanding of some of the physical and<br />
chemical properties of carotenoids allows analysts to<br />
determine carotenoids with greater ease and reliability.<br />
Solubility<br />
With very few exceptions, carotenoids are lipophilic.<br />
They are insoluble in water and soluble in organic<br />
solvents, such as acetone, alcohol, ethyl ether, chloroform,<br />
and ethyl acetate. Carotenes are readily<br />
soluble in petroleum ether, hexane, and toluene; xanthophylls<br />
dissolve better in methanol and ethanol.<br />
Crystalline carotenoids may be difficult to dissolve in<br />
the above solvents but do dissolve in benzene and<br />
dichloromethane (Schiedt and Liaaen-Jensen 1995).<br />
Solubility of both ß-carotene and the xanthophyll lutein<br />
in tetrahydrofuran was shown to be excellent (Craft<br />
and Soares 1992).<br />
Light Absorption<br />
The conjugated double-bond system constitutes the<br />
light-absorbing chromophore that gives carotenoids<br />
their attractive color and provides the visible<br />
absorption spectrum that serves as a basis for their<br />
identification and quantification. The color enables<br />
analysts to monitor the different steps of carotenoid<br />
analysis. Loss or change of color at any time during<br />
the analysis gives an immediate indication of<br />
degradation or structural modification. The color<br />
permits visual monitoring of the separation of<br />
carotenoids in open-column chromatography, and<br />
mainly for this reason this classical technique is still a<br />
viable option for quantitative analysis of carotenoids.<br />
The ultraviolet and visible spectrum is the first<br />
diagnostic tool for the identification of carotenoids.<br />
The wavelength of maximum absorption (λmax) and<br />
the shape of the spectrum (spectral fine structure)<br />
are characteristic of the chromophore. The structure-spectrum<br />
relationship has been extensively discussed.<br />
The λmax values of common carotenoids,<br />
taken mainly from Britton’s (1995) compilation, are<br />
shown in Table 7 and will be discussed in relation to<br />
the structures by using the absorption in petroleum<br />
ether.<br />
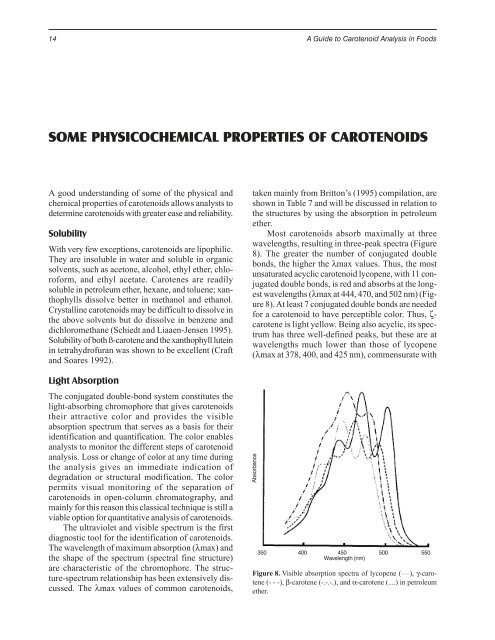
Most carotenoids absorb maximally at three<br />
wavelengths, resulting in three-peak spectra (Figure<br />
8). The greater the number of conjugated double<br />
bonds, the higher the λmax values. Thus, the most<br />
unsaturated acyclic carotenoid lycopene, with 11 conjugated<br />
double bonds, is red and absorbs at the longest<br />
wavelengths (λmax at 444, 470, and 502 nm) (Figure<br />
8). At least 7 conjugated double bonds are needed<br />
for a carotenoid to have perceptible color. Thus, ζcarotene<br />
is light yellow. Being also acyclic, its spectrum<br />
has three well-defined peaks, but these are at<br />
wavelengths much lower than those of lycopene<br />
(λmax at 378, 400, and 425 nm), commensurate with<br />
Absorbance<br />
350 400 450 500 550<br />
Wavelength (nm)<br />
Figure 8. Visible absorption spectra of lycopene ( ____ ), γ-carotene<br />
(- - -), β-carotene (-.-.-.), and α-carotene (....) in petroleum<br />
ether.