A GUIDE TO CAROTENOID ANALYSIS IN FOODS
A GUIDE TO CAROTENOID ANALYSIS IN FOODS
A GUIDE TO CAROTENOID ANALYSIS IN FOODS
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
NATURE OF <strong>CAROTENOID</strong>S <strong>IN</strong> <strong>FOODS</strong><br />
Food carotenoids are usually C 40 tetraterpenoids built<br />
from eight C 5 isoprenoid units, joined so that the<br />
sequence is reversed at the center. The basic linear<br />
and symmetrical skeleton, which can be cyclized at<br />
one or both ends, has lateral methyl groups separated<br />
by six C atoms at the center and five C atoms<br />
elsewhere. Cyclization and other modifications, such<br />
as hydrogenation, dehydrogenation, double-bond<br />
migration, chain shortening or extension,<br />
rearrangement, isomerization, introduction of oxygen<br />
functions, or combinations of these processes, result<br />
in a myriad of structures. A distinctive characteristic<br />
is an extensive conjugated double-bond system, which<br />
serves as the light-absorbing chromophore responsible<br />
for the yellow, orange, or red color that these<br />
compounds impart to many foods. Hydrocarbon<br />
carotenoids (i.e., carotenoids made up of only carbon<br />
and hydrogen) are collectively called carotenes; those<br />
containing oxygen are termed xanthophylls. In nature,<br />
they exist primarily in the more stable all-trans<br />
isomeric form, but cis isomers do occur. The first<br />
two C 40 carotenoids formed in the biosynthetic<br />
pathway have the 15-cis configuration in plants. The<br />
presence of small amounts of cis isomers of other<br />
carotenoids in natural sources has been increasingly<br />
reported.<br />
Because plants are able to synthesize carotenoids<br />
de novo, the carotenoid composition of plant foods is<br />
enriched by the presence of small or trace amounts<br />
of biosynthetic precursors, along with derivatives of<br />
the main components. Although commonly thought<br />
of as plant pigments, carotenoids are also encountered<br />
in some animal foods. Animals are incapable of<br />
carotenoid biosynthesis, thus their carotenoids are diet<br />
derived, selectively or unselectively absorbed, and<br />
accumulated unchanged or modified slightly into<br />
typical animal carotenoids.<br />
In the early stages of carotenoid biosynthesis, the<br />
C 5 primer for chain elongation undergoes successive<br />
additions of C 5 units, yielding in sequence C 10, C 15,<br />
and C 20 compounds. Dimerization of the latter<br />
produces phytoene, the first C 40 carotenoid. The<br />
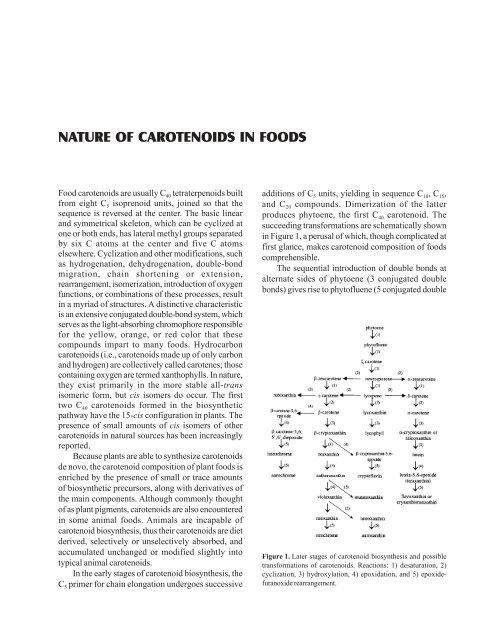
succeeding transformations are schematically shown<br />
in Figure 1, a perusal of which, though complicated at<br />
first glance, makes carotenoid composition of foods<br />
comprehensible.<br />
The sequential introduction of double bonds at<br />
alternate sides of phytoene (3 conjugated double<br />
bonds) gives rise to phytofluene (5 conjugated double<br />
Figure 1. Later stages of carotenoid biosynthesis and possible<br />
transformations of carotenoids. Reactions: 1) desaturation, 2)<br />
cyclization, 3) hydroxylation, 4) epoxidation, and 5) epoxidefuranoxide<br />
rearrangement.