A GUIDE TO CAROTENOID ANALYSIS IN FOODS
A GUIDE TO CAROTENOID ANALYSIS IN FOODS
A GUIDE TO CAROTENOID ANALYSIS IN FOODS
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
18 A Guide to Carotenoid Analysis in Foods<br />
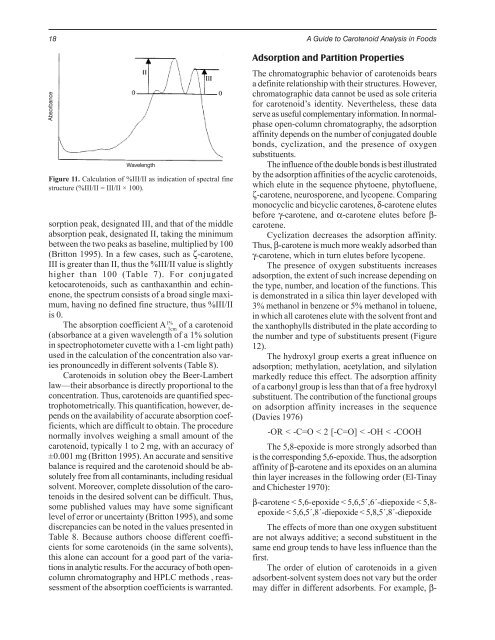
Absorbance<br />
sorption peak, designated III, and that of the middle<br />
absorption peak, designated II, taking the minimum<br />
between the two peaks as baseline, multiplied by 100<br />
(Britton 1995). In a few cases, such as ζ-carotene,<br />
III is greater than II, thus the %III/II value is slightly<br />
higher than 100 (Table 7). For conjugated<br />
ketocarotenoids, such as canthaxanthin and echinenone,<br />
the spectrum consists of a broad single maximum,<br />
having no defined fine structure, thus %III/II<br />
is 0.<br />
Wavelength<br />
Figure 11. Calculation of %III/II as indication of spectral fine<br />
structure (%III/II = III/II × 100).<br />
The absorption coefficient A1% of a carotenoid<br />
1cm<br />
(absorbance at a given wavelength of a 1% solution<br />
in spectrophotometer cuvette with a 1-cm light path)<br />
used in the calculation of the concentration also varies<br />
pronouncedly in different solvents (Table 8).<br />
Carotenoids in solution obey the Beer-Lambert<br />
law—their absorbance is directly proportional to the<br />
concentration. Thus, carotenoids are quantified spectrophotometrically.<br />
This quantification, however, depends<br />
on the availability of accurate absorption coefficients,<br />
which are difficult to obtain. The procedure<br />
normally involves weighing a small amount of the<br />
carotenoid, typically 1 to 2 mg, with an accuracy of<br />
±0.001 mg (Britton 1995). An accurate and sensitive<br />
balance is required and the carotenoid should be absolutely<br />
free from all contaminants, including residual<br />
solvent. Moreover, complete dissolution of the carotenoids<br />
in the desired solvent can be difficult. Thus,<br />
some published values may have some significant<br />
level of error or uncertainty (Britton 1995), and some<br />
discrepancies can be noted in the values presented in<br />
Table 8. Because authors choose different coefficients<br />
for some carotenoids (in the same solvents),<br />
this alone can account for a good part of the variations<br />
in analytic results. For the accuracy of both opencolumn<br />
chromatography and HPLC methods , reassessment<br />
of the absorption coefficients is warranted.<br />
Adsorption and Partition Properties<br />
The chromatographic behavior of carotenoids bears<br />
a definite relationship with their structures. However,<br />
chromatographic data cannot be used as sole criteria<br />
for carotenoid’s identity. Nevertheless, these data<br />
serve as useful complementary information. In normalphase<br />
open-column chromatography, the adsorption<br />
affinity depends on the number of conjugated double<br />
bonds, cyclization, and the presence of oxygen<br />
substituents.<br />
The influence of the double bonds is best illustrated<br />
by the adsorption affinities of the acyclic carotenoids,<br />
which elute in the sequence phytoene, phytofluene,<br />
ζ-carotene, neurosporene, and lycopene. Comparing<br />
monocyclic and bicyclic carotenes, δ-carotene elutes<br />
before γ-carotene, and α-carotene elutes before βcarotene.<br />
Cyclization decreases the adsorption affinity.<br />
Thus, β-carotene is much more weakly adsorbed than<br />
γ-carotene, which in turn elutes before lycopene.<br />
The presence of oxygen substituents increases<br />
adsorption, the extent of such increase depending on<br />
the type, number, and location of the functions. This<br />
is demonstrated in a silica thin layer developed with<br />
3% methanol in benzene or 5% methanol in toluene,<br />
in which all carotenes elute with the solvent front and<br />
the xanthophylls distributed in the plate according to<br />
the number and type of substituents present (Figure<br />
12).<br />
The hydroxyl group exerts a great influence on<br />
adsorption; methylation, acetylation, and silylation<br />
markedly reduce this effect. The adsorption affinity<br />
of a carbonyl group is less than that of a free hydroxyl<br />
substituent. The contribution of the functional groups<br />
on adsorption affinity increases in the sequence<br />
(Davies 1976)<br />
-OR < -C=O < 2 [-C=O] < -OH < -COOH<br />
The 5,8-epoxide is more strongly adsorbed than<br />
is the corresponding 5,6-epoxide. Thus, the adsorption<br />
affinity of β-carotene and its epoxides on an alumina<br />
thin layer increases in the following order (El-Tinay<br />
and Chichester 1970):<br />
β-carotene < 5,6-epoxide < 5,6,5´,6´-diepoxide < 5,8epoxide<br />
< 5,6,5´,8´-diepoxide < 5,8,5´,8´-diepoxide<br />
The effects of more than one oxygen substituent<br />
are not always additive; a second substituent in the<br />
same end group tends to have less influence than the<br />
first.<br />
The order of elution of carotenoids in a given<br />
adsorbent-solvent system does not vary but the order<br />
may differ in different adsorbents. For example, β-