A GUIDE TO CAROTENOID ANALYSIS IN FOODS
A GUIDE TO CAROTENOID ANALYSIS IN FOODS
A GUIDE TO CAROTENOID ANALYSIS IN FOODS
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
44 A Guide to Carotenoid Analysis in Foods<br />
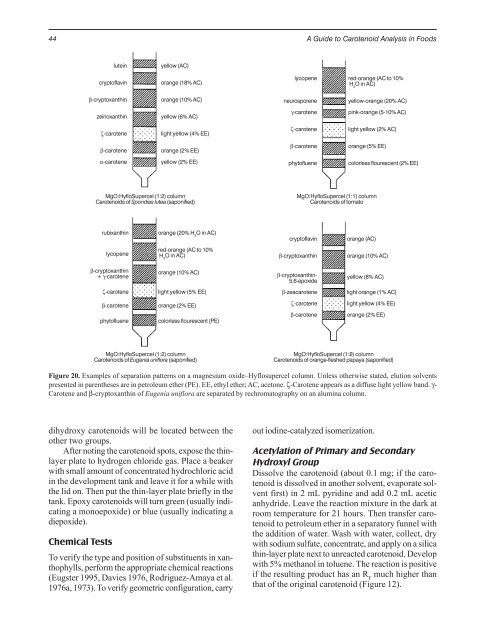
Figure 20. Examples of separation patterns on a magnesium oxide–Hyflosupercel column. Unless otherwise stated, elution solvents<br />
presented in parentheses are in petroleum ether (PE). EE, ethyl ether; AC, acetone. ζ-Carotene appears as a diffuse light yellow band. γ-<br />
Carotene and β-cryptoxanthin of Eugenia uniflora are separated by rechromatography on an alumina column.<br />
dihydroxy carotenoids will be located between the<br />
other two groups.<br />
After noting the carotenoid spots, expose the thinlayer<br />
plate to hydrogen chloride gas. Place a beaker<br />
with small amount of concentrated hydrochloric acid<br />
in the development tank and leave it for a while with<br />
the lid on. Then put the thin-layer plate briefly in the<br />
tank. Epoxy carotenoids will turn green (usually indicating<br />
a monoepoxide) or blue (usually indicating a<br />
diepoxide).<br />
Chemical Tests<br />
lutein<br />
cryptoflavin<br />
β-cryptoxanthin<br />
zeinoxanthin<br />
ζ-carotene<br />
β-carotene<br />
α-carotene<br />
yellow (AC)<br />
orange (18% AC)<br />
orange (10% AC)<br />
yellow (6% AC)<br />
light yellow (4% EE)<br />
orange (2% EE)<br />
yellow (2% EE)<br />
MgO:HyfloSupercel (1:2) column<br />
Carotenoids of Spondias lutea (saponified)<br />
rubixanthin<br />
lycopene<br />
β-cryptoxanthin<br />
+ γ-carotene<br />
ζ-carotene<br />
β-carotene<br />
phytofluene<br />
orange (20% H 2 O in AC)<br />
red-orange (AC to 10%<br />
H 2 O in AC)<br />
orange (10% AC)<br />
light yellow (5% EE)<br />
orange (2% EE)<br />
colorless flourescent (PE)<br />
MgO:HyfloSupercel (1:2) column<br />
Carotenoids of Eugenia uniflora (saponified)<br />
To verify the type and position of substituents in xanthophylls,<br />
perform the appropriate chemical reactions<br />
(Eugster 1995, Davies 1976, Rodriguez-Amaya et al.<br />
1976a, 1973). To verify geometric configuration, carry<br />
lycopene<br />
neurosporene<br />
γ-carotene<br />
ζ-carotene<br />
β-carotene<br />
phytofluene<br />
out iodine-catalyzed isomerization.<br />
red-orange (AC to 10%<br />
H 2 O in AC)<br />
yellow-orange (20% AC)<br />
pink-orange (5-10% AC)<br />
light yellow (2% AC)<br />
orange (5% EE)<br />
MgO:HyfloSupercel (1:1) column<br />
Carotenoids of tomato<br />
cryptoflavin<br />
β-cryptoxanthin<br />
β-cryptoxanthin-<br />
5,6-epoxide<br />
β-zeacarotene<br />
ζ-carotene<br />
β-carotene<br />
colorless flourescent (2% EE)<br />
orange (AC)<br />
orange (10% AC)<br />
yellow (8% AC)<br />
light orange (1% AC)<br />
light yellow (4% EE)<br />
orange (2% EE)<br />
MgO:HyfloSupercel (1:2) column<br />
Carotenoids of orange-fleshed papaya (saponified)<br />
Acetylation of Primary and Secondary<br />
Hydroxyl Group<br />
Dissolve the carotenoid (about 0.1 mg; if the carotenoid<br />
is dissolved in another solvent, evaporate solvent<br />
first) in 2 mL pyridine and add 0.2 mL acetic<br />
anhydride. Leave the reaction mixture in the dark at<br />
room temperature for 21 hours. Then transfer carotenoid<br />
to petroleum ether in a separatory funnel with<br />
the addition of water. Wash with water, collect, dry<br />
with sodium sulfate, concentrate, and apply on a silica<br />
thin-layer plate next to unreacted carotenoid. Develop<br />
with 5% methanol in toluene. The reaction is positive<br />
if the resulting product has an R F much higher than<br />
that of the original carotenoid (Figure 12).