Catarina Maia Seco Seiça Neves Sistemas Aquosos Bifásicos com ...
Catarina Maia Seco Seiça Neves Sistemas Aquosos Bifásicos com ...
Catarina Maia Seco Seiça Neves Sistemas Aquosos Bifásicos com ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Aqueous Biphasic Systems with Ionic Liquids<br />
_________________________________________________________________________<br />
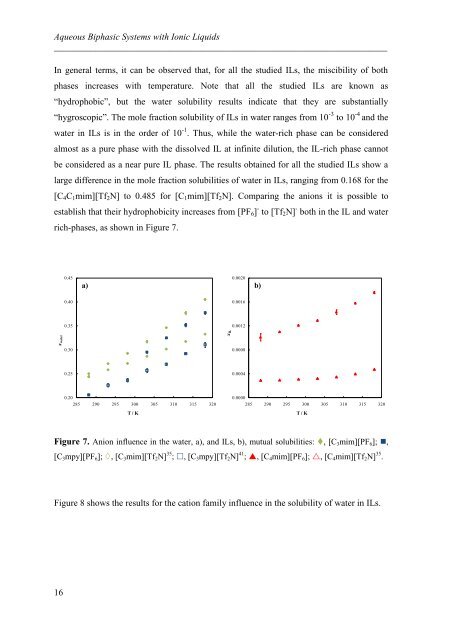
In general terms, it can be observed that, for all the studied ILs, the miscibility of both<br />
phases increases with temperature. Note that all the studied ILs are known as<br />
“hydrophobic”, but the water solubility results indicate that they are substantially<br />
“hygroscopic”. The mole fraction solubility of ILs in water ranges from 10 -3 to 10 -4 and the<br />
water in ILs is in the order of 10 -1 . Thus, while the water-rich phase can be considered<br />
almost as a pure phase with the dissolved IL at infinite dilution, the IL-rich phase cannot<br />
be considered as a near pure IL phase. The results obtained for all the studied ILs show a<br />
large difference in the mole fraction solubilities of water in ILs, ranging from 0.168 for the<br />
[C4C1mim][Tf2N] to 0.485 for [C1mim][Tf2N]. Comparing the anions it is possible to<br />
establish that their hydrophobicity increases from [PF6] - to [Tf2N] - both in the IL and water<br />
rich-phases, as shown in Figure 7.<br />
x water<br />
Figure 7. Anion influence in the water, a), and ILs, b), mutual solubilities: , [C3mim][PF6]; ,<br />
[C3mpy][PF6]; , [C3mim][Tf2N] 35 ; , [C3mpy][Tf2N] 41 ; , [C4mim][PF6]; , [C4mim][Tf2N] 35 .<br />
Figure 8 shows the results for the cation family influence in the solubility of water in ILs.<br />
16<br />
0.45<br />
0.40<br />
0.35<br />
0.30<br />
0.25<br />
0.20<br />
285 290 295 300 305 310 315 320<br />
x IL<br />
0.0020<br />
a) b)<br />
T / K<br />
0.0016<br />
0.0012<br />
0.0008<br />
0.0004<br />
0.0000<br />
285 290 295 300 305 310 315 320<br />
T / K