S3-Guideline “Exocrine Pancreatic Carcinoma” 20071 ... - DGVS
S3-Guideline “Exocrine Pancreatic Carcinoma” 20071 ... - DGVS
S3-Guideline “Exocrine Pancreatic Carcinoma” 20071 ... - DGVS
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
452<br />
Leitlinie<br />
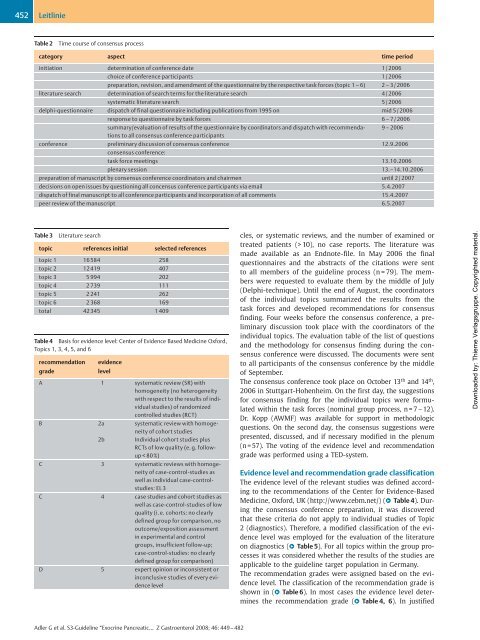
Table 2 Time course of consensus process<br />
category aspect time period<br />
initiation determination of conference date 1/2006<br />
choice of conference participants 1/2006<br />
preparation, revision, and amendment of the questionnaire by the respective task forces (topic 1 – 6) 2 – 3/2006<br />
literature search determination of search terms for the literature search 4/2006<br />
systematic literature search 5/2006<br />
delphi-questionnaire dispatch of final questionnaire including publications from 1995 on mid 5/2006<br />
response to questionnaire by task forces 6 – 7/2006<br />
summary/evaluation of results of the questionnaire by coordinators and dispatch with recommendations<br />
to all consensus conference participants<br />
9 – 2006<br />
conference preliminary discussion of consensus conference<br />
consensus conference:<br />
12.9.2006<br />
task force meetings 13.10.2006<br />
plenary session 13.–14.10.2006<br />
preparation of manuscript by consensus conference coordinators and chairmen until 2/2007<br />
decisions on open issues by questioning all concensus conference participants via email 5.4.2007<br />
dispatch of final manuscript to all conference participants and incorporation of all comments 15.4.2007<br />
peer review of the manuscript 6.5.2007<br />
Table 3 Literature search<br />
topic references initial selected references<br />
topic 1 16584 258<br />
topic 2 12419 407<br />
topic 3 5 994 202<br />
topic 4 2 739 111<br />
topic 5 2 241 262<br />
topic 6 2 368 169<br />
total 42345 1 409<br />
Table 4 Basis for evidence level: Center of Evidence Based Medicine Oxford,<br />
Topics 1, 3, 4, 5, and 6<br />
recommendation<br />
grade<br />
evidence<br />
level<br />
A 1 systematic review (SR) with<br />
homogeneity (no heterogeneity<br />
with respect to the results of individual<br />
studies) of randomized<br />
controlled studies (RCT)<br />
B 2a<br />
systematic review with homogeneity<br />
of cohort studies<br />
2b<br />
Individual cohort studies plus<br />
RCTs of low quality (e. g. followup<br />
< 80%)<br />
C 3 systematic reviews with homogeneity<br />
of case-control-studies as<br />
well as individual case-controlstudies:<br />
EL 3<br />
C 4 case studies and cohort studies as<br />
well as case-control-studies of low<br />
quality (i. e. cohorts: no clearly<br />
defined group for comparison, no<br />
outcome/exposition assessment<br />
in experimental and control<br />
groups, insufficient follow-up;<br />
case-control-studies: no clearly<br />
defined group for comparison)<br />
D 5 expert opinion or inconsistent or<br />
inconclusive studies of every evidence<br />
level<br />
Adler G et al. <strong>S3</strong>-<strong>Guideline</strong> <strong>“Exocrine</strong> <strong>Pancreatic</strong>… Z Gastroenterol 2008; 46: 449–482<br />
cles, or systematic reviews, and the number of examined or<br />
treated patients (> 10), no case reports. The literature was<br />
made available as an Endnote-file. In May 2006 the final<br />
questionnaires and the abstracts of the citations were sent<br />
to all members of the guideline process (n = 79). The members<br />
were requested to evaluate them by the middle of July<br />
(Delphi-technique). Until the end of August, the coordinators<br />
of the individual topics summarized the results from the<br />
task forces and developed recommendations for consensus<br />
finding. Four weeks before the consensus conference, a preliminary<br />
discussion took place with the coordinators of the<br />
individual topics. The evaluation table of the list of questions<br />
and the methodology for consensus finding during the consensus<br />
conference were discussed. The documents were sent<br />
to all participants of the consensus conference by the middle<br />
of September.<br />
The consensus conference took place on October 13 th and 14 th ,<br />
2006 in Stuttgart-Hohenheim. On the first day, the suggestions<br />
for consensus finding for the individual topics were formulated<br />
within the task forces (nominal group process, n = 7 – 12).<br />
Dr. Kopp (AWMF) was available for support in methodologic<br />
questions. On the second day, the consensus suggestions were<br />
presented, discussed, and if necessary modified in the plenum<br />
(n = 57). The voting of the evidence level and recommendation<br />
grade was performed using a TED-system.<br />
Evidence level and recommendation grade classification<br />
The evidence level of the relevant studies was defined according<br />
to the recommendations of the Center for Evidence-Based<br />
Medicine, Oxford, UK (http://www.cebm.net/) (l " Table 4). During<br />
the consensus conference preparation, it was discovered<br />
that these criteria do not apply to individual studies of Topic<br />
2 (diagnostics). Therefore, a modified classification of the evidence<br />
level was employed for the evaluation of the literature<br />
on diagnostics (l " Table 5). For all topics within the group processes<br />
it was considered whether the results of the studies are<br />
applicable to the guideline target population in Germany.<br />
The recommendation grades were assigned based on the evidence<br />
level. The classification of the recommendation grade is<br />
shown in (l " Table 6). In most cases the evidence level determines<br />
the recommendation grade (l " Table 4, 6). In justified<br />
Downloaded by: Thieme Verlagsgruppe. Copyrighted material.