Chapter 9 - University of Dayton Academic Webserver
Chapter 9 - University of Dayton Academic Webserver
Chapter 9 - University of Dayton Academic Webserver
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
294<br />
..<br />
. F..<br />
. . .<br />
O.<br />
.<br />
<strong>Chapter</strong> 9: Molecular Structures<br />
. .<br />
.<br />
F<br />
S<br />
. .<br />
. F.<br />
. .<br />
. F.<br />
120¡<br />
F<br />
O<br />
90¡<br />
F<br />
S F<br />
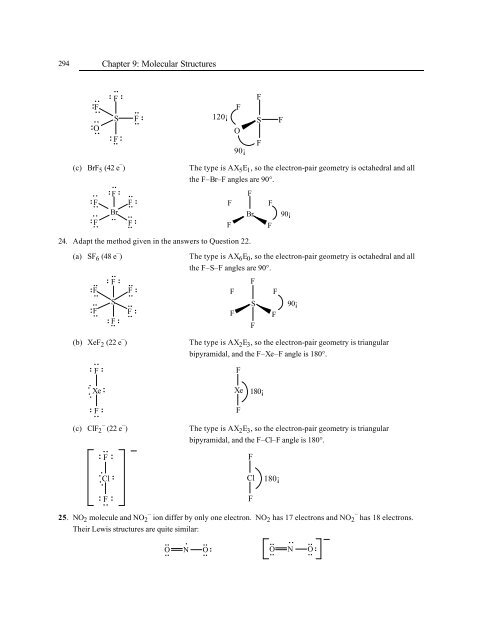
(c) BrF 5 (42 e – ) The type is AX 5E 1, so the electron-pair geometry is octahedral and all<br />
..<br />
. ..<br />
. . .<br />
. F.<br />
. . .<br />
.<br />
. . .<br />
. .<br />
..<br />
.. .<br />
F<br />
the F–Br–F angles are 90°.<br />
F<br />
F<br />
F F<br />
F F<br />
Br<br />
. .<br />
F F<br />
Br<br />
F<br />
24. Adapt the method given in the answers to Question 22.<br />
(a) SF 6 (48 e – ) The type is AX 6E 0, so the electron-pair geometry is octahedral and all<br />
. . .<br />
. .<br />
..<br />
. .. F<br />
..<br />
. .<br />
S<br />
.<br />
. F.<br />
.<br />
..<br />
.. .<br />
. . .<br />
. .<br />
F<br />
F F<br />
the F–S–F angles are 90°.<br />
F F<br />
F<br />
F F<br />
(b) XeF 2 (22 e – ) The type is AX 2E 3, so the electron-pair geometry is triangular<br />
. . .<br />
F .<br />
..<br />
.<br />
. Xe .<br />
.. F . .<br />
S<br />
F<br />
F<br />
90¡<br />
90¡<br />
bipyramidal, and the F–Xe–F angle is 180°.<br />
(c) ClF 2 – (22 e – ) The type is AX 2E 3, so the electron-pair geometry is triangular<br />
. . .<br />
F .<br />
..<br />
..<br />
. Cl<br />
.. F . .<br />
F<br />
Xe<br />
F<br />
180¡<br />
bipyramidal, and the F–Cl–F angle is 180°.<br />
25. NO 2 molecule and NO 2 – ion differ by only one electron. NO2 has 17 electrons and NO 2 – has 18 electrons.<br />
Their Lewis structures are quite similar:<br />
.. .<br />
..<br />
..<br />
.. .<br />
F<br />
Cl<br />
F<br />
180¡<br />
. . . . . .<br />
. . . .<br />
O N O O N O .